Molecular basis of positive allosteric modulation of GluN2B NMDA receptors by polyamines
- PMID: 21685875
- PMCID: PMC3160180
- DOI: 10.1038/emboj.2011.203
Molecular basis of positive allosteric modulation of GluN2B NMDA receptors by polyamines
Abstract
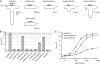
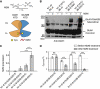
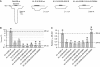
NMDA receptors (NMDARs) form glutamate-gated ion channels that have central roles in neuronal communication and plasticity throughout the brain. Dysfunctions of NMDARs are involved in several central nervous system disorders, including stroke, chronic pain and schizophrenia. One hallmark of NMDARs is that their activity can be allosterically regulated by a variety of extracellular small ligands. While much has been learned recently regarding allosteric inhibition of NMDARs, the structural determinants underlying positive allosteric modulation of these receptors remain poorly defined. Here, we show that polyamines, naturally occurring polycations that selectively enhance NMDARs containing the GluN2B subunit, bind at a dimer interface between GluN1 and GluN2B subunit N-terminal domains (NTDs). Polyamines act by shielding negative charges present on GluN1 and GluN2B NTD lower lobes, allowing their close apposition, an effect that in turn prevents NTD clamshell closure. Our work reveals the mechanistic basis for positive allosteric modulation of NMDARs. It provides the first example of an intersubunit binding site in this class of receptors, a discovery that holds promise for future drug interventions.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
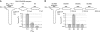


References
-
- Armstrong N, Jasti J, Beich-Frandsen M, Gouaux E (2006) Measurement of conformational changes accompanying desensitization in an ionotropic glutamate receptor. Cell 127: 85–97 - PubMed
-
- Bertrand D, Gopalakrishnan M (2007) Allosteric modulation of nicotinic acetylcholine receptors. Biochem Pharmacol 74: 1155–1163 - PubMed
-
- Chesler M, Kaila K (1992) Modulation of pH by neuronal activity. Trends Neurosci 15: 396–402 - PubMed
-
- Choi YB, Lipton SA (1999) Identification and mechanism of action of two histidine residues underlying high-affinity Zn2+ inhibition of the NMDA receptor. Neuron 23: 171–180 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

