Broad antigenic coverage induced by vaccination with virus-based cDNA libraries cures established tumors
- PMID: 21685898
- PMCID: PMC3918897
- DOI: 10.1038/nm.2390
Broad antigenic coverage induced by vaccination with virus-based cDNA libraries cures established tumors
Erratum in
- Nat Med. 2012 Sep;18(9):1445
Abstract
Effective cancer immunotherapy requires the release of a broad spectrum of tumor antigens in the context of potent immune activation. We show here that a cDNA library of normal tissue, expressed from a highly immunogenic viral platform, cures established tumors of the same histological type from which the cDNA library was derived. Immune escape occurred with suboptimal vaccination, but tumor cells that escaped the immune pressure were readily treated by second-line virus-based immunotherapy. This approach has several major advantages. Use of the cDNA library leads to presentation of a broad repertoire of (undefined) tumor-associated antigens, which reduces emergence of treatment-resistant variants and also permits rational, combined-modality approaches in the clinic. Finally, the viral vectors can be delivered systemically, without the need for tumor targeting, and are amenable to clinical-grade production. Therefore, virus-expressed cDNA libraries represent a novel paradigm for cancer treatment addressing many of the key issues that have undermined the efficacy of immuno- and virotherapy to date.
Figures
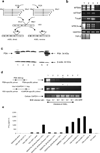
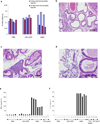
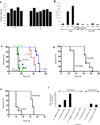

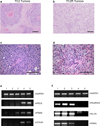


Comment in
-
A viral strategy to ambush tumors.Nat Med. 2011 Jul 7;17(7):784-5. doi: 10.1038/nm0711-784. Nat Med. 2011. PMID: 21738155 Free PMC article.
-
Immunotherapy: A vaccine for prostate cancer?Nat Rev Cancer. 2011 Jul 22;11(8):539. doi: 10.1038/nrc3117. Nat Rev Cancer. 2011. PMID: 21779008 No abstract available.
-
Cancer: vaccine cure for prostate cancer?Nat Rev Drug Discov. 2011 Aug 1;10(8):575. doi: 10.1038/nrd3524. Nat Rev Drug Discov. 2011. PMID: 21804590 No abstract available.
-
Prostate cancer: cDNA vaccination using a viral vector can cure prostate tumors.Nat Rev Urol. 2011 Aug 10;8(8):411. doi: 10.1038/nrurol.2011.105. Nat Rev Urol. 2011. PMID: 21829234 No abstract available.
References
-
- Drake CG, Jaffee EM, Pardoll DM. Mechanisms of immune evasion by tumors. Adv. Immunol. 2006;90:51–81. - PubMed
-
- Koos D, et al. Tumor vaccines in 2010: need for integration. Cell. Immunol. 2010;263:138–147. - PubMed
-
- Parato KA, Lichty BD, Bell JC. Diplomatic immunity: turning a foe into an ally. Curr. Opin. Mol. Ther. 2009;11:13–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

