Nanoparticles that communicate in vivo to amplify tumour targeting
- PMID: 21685903
- PMCID: PMC3361766
- DOI: 10.1038/nmat3049
Nanoparticles that communicate in vivo to amplify tumour targeting
Abstract
Nanomedicines have enormous potential to improve the precision of cancer therapy, yet our ability to efficiently home these materials to regions of disease in vivo remains very limited. Inspired by the ability of communication to improve targeting in biological systems, such as inflammatory-cell recruitment to sites of disease, we construct systems where synthetic biological and nanotechnological components communicate to amplify disease targeting in vivo. These systems are composed of 'signalling' modules (nanoparticles or engineered proteins) that target tumours and then locally activate the coagulation cascade to broadcast tumour location to clot-targeted 'receiving' nanoparticles in circulation that carry a diagnostic or therapeutic cargo, thereby amplifying their delivery. We show that communicating nanoparticle systems can be composed of multiple types of signalling and receiving modules, can transmit information through multiple molecular pathways in coagulation, can operate autonomously and can target over 40 times higher doses of chemotherapeutics to tumours than non-communicating controls.
© 2011 Macmillan Publishers Limited. All rights reserved
Figures
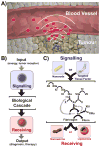

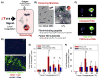
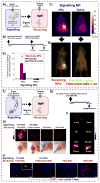

Comment in
-
Nanomedicine: swarming towards the target.Nat Mater. 2011 Jun 19;10(7):482-3. doi: 10.1038/nmat3060. Nat Mater. 2011. PMID: 21685899 No abstract available.
-
Nanotechnology: Tag teams.Nat Rev Cancer. 2011 Jul 14;11(8):537. doi: 10.1038/nrc3111. Nat Rev Cancer. 2011. PMID: 21753792 No abstract available.
References
-
- Chan WC, Nie S. Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science. 1998;281:2016–2018. - PubMed
-
- Xia YN, Halas NJ. Shape-controlled synthesis and surface plasmonic properties of metallic nanostructures. Mrs Bulletin. 2005;30:338–344.
-
- Gref R, et al. Biodegradable long-circulating polymeric nanospheres. Science. 1994;263:1600–1603. - PubMed
-
- Sengupta S, et al. Temporal targeting of tumour cells and neovasculature with a nanoscale delivery system. Nature. 2005;436:568–572. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

