Perforin pores in the endosomal membrane trigger the release of endocytosed granzyme B into the cytosol of target cells
- PMID: 21685908
- PMCID: PMC3140544
- DOI: 10.1038/ni.2050
Perforin pores in the endosomal membrane trigger the release of endocytosed granzyme B into the cytosol of target cells
Abstract
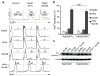
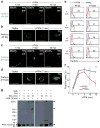
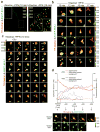
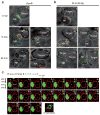
How the pore-forming protein perforin delivers apoptosis-inducing granzymes to the cytosol of target cells is uncertain. Perforin induces a transient Ca2+ flux in the target cell, which triggers a process to repair the damaged cell membrane. As a consequence, both perforin and granzymes are endocytosed into enlarged endosomes called 'gigantosomes'. Here we show that perforin formed pores in the gigantosome membrane, allowing endosomal cargo, including granzymes, to be gradually released. After about 15 min, gigantosomes ruptured, releasing their remaining content. Thus, perforin delivers granzymes by a two-step process that involves first transient pores in the cell membrane that trigger the endocytosis of granzyme and perforin and then pore formation in endosomes to trigger cytosolic release.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


Comment in
-
Endocytosis by target cells: an essential means for perforin- and granzyme-mediated killing.Cell Mol Immunol. 2012 Jan;9(1):5-6. doi: 10.1038/cmi.2011.45. Epub 2011 Sep 19. Cell Mol Immunol. 2012. PMID: 21927017 Free PMC article. No abstract available.
References
-
- de Saint Basile G, Menasche G, Fischer A. Molecular mechanisms of biogenesis and exocytosis of cytotoxic granules. Nat Rev Immunol. 2010;10:568–579. - PubMed
-
- Grakoui A, et al. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–227. - PubMed
-
- Stinchcombe JC, Bossi G, Booth S, Griffiths GM. The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity. 2001;15:751–761. - PubMed
-
- Lieberman J. The ABCs of granule-mediated cytotoxicity: new weapons in the arsenal. Nat Rev Immunol. 2003;3:361–370. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

