Regulation of the activation of the Fanconi anemia pathway by the p21 cyclin-dependent kinase inhibitor
- PMID: 21685936
- PMCID: PMC3974337
- DOI: 10.1038/onc.2011.237
Regulation of the activation of the Fanconi anemia pathway by the p21 cyclin-dependent kinase inhibitor
Abstract
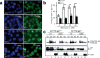
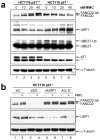
Fanconi anemia (FA) is a rare disease characterized by congenital defects, progressive bone marrow failure and heightened cancer susceptibility. The FA proteins, BRCA1 and FANCD1/BRCA2 function cooperatively in the FA-BRCA pathway to repair damaged DNA. Activation of the FA-BRCA pathway occurs via the monoubiquitination of the FANCD2 and FANCI proteins, targeting these proteins to discrete nuclear foci where they function in DNA repair. The cellular regulation of FANCD2/I monoubiquitination, however, remains poorly understood. In this study, we have examined the roles of the p53 tumor suppressor protein, as well as its downstream target, the p21(Cip1/Waf1) cyclin-dependent kinase inhibitor, in the regulation of the activation of the FA-BRCA pathway. We demonstrate that, in contrast to p53, p21 has a major role in the regulation of the activation of the FA-BRCA pathway: p21 promotes S-phase and DNA damage-inducible FANCD2/I monoubiquitination and nuclear foci formation. Several lines of evidence establish that this effect is not a consequence of a defective G1-S checkpoint or altered cell-cycle progression in the absence of p21. Instead, we demonstrate that p21 is required for the transcriptional repression of the USP1 deubiquitinating enzyme upon exposure to DNA-damaging agents. In the absence of p21, persistent USP1 expression precludes the DNA damage-inducible accumulation of monoubiquitinated FANCD2 and FANCI. Consequently, p21(-/-) cells exhibit increased levels of mitomycin C-inducible complex chromosomal aberrations and elevated γH2AX nuclear foci formation. Our results demonstrate that p21 has a critical role in the regulation of the activation of the FA-BRCA pathway and suggest a broader role for p21 in the orchestration of DNA repair processes following exposure to DNA crosslinking agents.
Conflict of interest statement
There are no competing financial interests in relation to the work described.
Figures




References
-
- Adamo A, Collis SJ, Adelman CA, Silva N, Horejsi Z, Ward JD, et al. Preventing nonhomologous end joining suppresses DNA repair defects of Fanconi anemia. Mol Cell. 2010;39:25–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

