Stem cell marker (Nanog) and Stat-3 signaling promote MicroRNA-21 expression and chemoresistance in hyaluronan/CD44-activated head and neck squamous cell carcinoma cells
- PMID: 21685938
- PMCID: PMC3179812
- DOI: 10.1038/onc.2011.222
Stem cell marker (Nanog) and Stat-3 signaling promote MicroRNA-21 expression and chemoresistance in hyaluronan/CD44-activated head and neck squamous cell carcinoma cells
Abstract
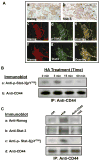
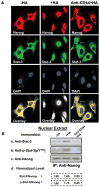
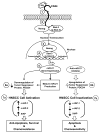
MicroRNAs are often associated with the pathogenesis of many cancers, including head and neck squamous cell carcinoma (HNSCC). In particular, microRNA-21 (miR-21) appears to have a critical role in tumor cell survival, chemoresistance and HNSCC progression. In this study, we investigated matrix hyaluronan (HA)-induced CD44 (a primary HA receptor) interaction with the stem cell markers, Nanog and Stat-3, in HNSCC cells (HSC-3 cells). Our results indicate that HA binding to CD44 promotes Nanog-Stat-3 (also tyrosine phosphorylated Stat-3) complex formation, nuclear translocation and transcriptional activation. Further analyses reveal that miR-21 is controlled by an upstream promoter containing Stat-3 binding site(s), while chromatin immunoprecipitation assays demonstrate that stimulation of miR-21 expression by HA/CD44 signaling is Nanog/Stat-3-dependent in HNSCC cells. This process results in a decrease of a tumor suppressor protein (PDCD4), and an upregulation of i nhibitors of the apoptosis family of proteins (IAPs) as well as chemoresistance in HSC-3 cells. Treatment of HSC-3 cells with Nanog- and/or Stat-3-specific small interfering RNAs effectively blocks HA-mediated Nanog-Stat-3 signaling events, abrogates miR-21 production and increases PDCD4 expression. Subsequently, this Nanog-Stat-3 signaling inhibition causes downregulation of survival protein (IAP) expression and enhancement of chemosensitivity. To further evaluate the role of miR-21 in tumor cell-specific functions, HSC-3 cells were also transfected with a specific anti-miR-21 inhibitor in order to silence miR-21 expression and block its target functions. Our results demonstrate that anti-miR-21 inhibitor not only upregulates PDCD4 expression but also decreases IAP expression and enhances chemosensitivity in HA-treated HNSCC cells. Together, these findings indicate that the HA-induced CD44 interaction with Nanog and Stat-3 has a pivotal role in miR-21 production leading to PDCD4 reduction, IAP upregulation and chemoresistance in HNSCC cells. This novel Nanog/Stat-3 signaling pathway-specific mechanism involved in miR-21 production is significant for the formation of future intervention strategies in the treatment of HA/CD44-activated HNSCC.
Conflict of interest statement
Figures



Comment in
-
Hyaluronan-CD44 interaction promotes microRNA signaling and RhoGTPase activation leading to tumor progression.Small GTPases. 2012 Jan-Mar;3(1):53-9. doi: 10.4161/sgtp.19110. Small GTPases. 2012. PMID: 22714418 Free PMC article.
References
-
- Asangani IA, Rasheed SAK, Nikolova DA, Leupold JH, Colburn NH, Post S, et al. Allgayer H. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. 2008;27:2128–2136. - PubMed
-
- Bourguignon LY, Spevak CC, Wong G, Xia W, Gilad E. Hyaluronan-CD44 interaction with PKCε promotes oncogenic signaling by the stem cell marker, Nanog and the production of microRNA-21 leading to downregulation of the tumor suppressor protein, PDCD4, anti-apoptosis and chemotherapy resistance in breast tumor cells. J Biol Chem. 2009a;284:26533–26546. - PMC - PubMed
-
- Bourguignon LY, Xia W, Wong G. Hyaluronan-mediated CD44 interaction with p300 and SIRT1 regulates beta-catenin signaling and NFkappaB-specific transcription activity leading to MDR1 and Bcl-xL gene expression and chemoresistance in breast tumor cells. J Biol Chem. 2009b;284:2657–2671. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

