Chemoenzymatic synthesis of α2-3-sialylated carbohydrate epitopes
- PMID: 21686057
- PMCID: PMC3115702
- DOI: 10.1007/s11426-010-4175-9
Chemoenzymatic synthesis of α2-3-sialylated carbohydrate epitopes
Abstract
Sialic acids are common terminal carbohydrates on cell surface. Together with internal carbohydrate structures, they play important roles in many physiological and pathological processes. In order to obtain α2-3-sialylated oligosaccharides, a highly efficient one-pot three-enzyme synthetic approach was applied. The P. multocida α2-3-sialyltransferase (PmST1) involved in the synthesis was a multifunctional enzyme with extremely flexible donor and acceptor substrate specificities. Sialyltransferase acceptors, including type 1 structure (Galβ1-3GlcNAcβProN(3)), type 2 structures (Galβ1-4GlcNAcβProN(3) and 6-sulfo-Galβ1-4GlcNAcβProN(3)), type 4 structure (Galβ1-3GalNAcβProN(3)), type 3 or core 1 structure (Galβ1-3GalNAcαProN(3)) and human milk oligosaccharide or lipooligosaccharide lacto-N-tetraose (LNT) (Galβ1-3GlcNAcβ1-3Galβ1-4GlcβProN(3)), were chemically synthesized. They were then used in one-pot three-enzyme reactions with sialic acid precursor ManNAc or ManNGc, to synthesize a library of natural occurring α2-3-linked sialosides with different internal sugar units. The sialylated oligosaccharides obtained are valuable probes for their biological studies.
Figures

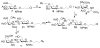



References
-
- Tsuji S, Datta AK, Paulson JC. Systematic nomenclature for sialyltransferases. Glycobiology. 1996;6:v–vii. - PubMed
-
- Fukuda MN, Dell A, Oates JE, Fukuda M. Embryonal lactosaminoglycan. The structure of branched lactosaminoglycans with novel disialosyl (sialyl alpha 2–9 sialyl) terminals isolated from PA1 human embryonal carcinoma cells. J Biol Chem. 1985;260:6623–6631. - PubMed
-
- Angata T, Varki A. Chemical diversity in the sialic acids and related alpha-keto acids: an evolutionary perspective. Chem Rev. 2002;102:439–469. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
