Antioxidant properties of aminoethylcysteine ketimine decarboxylated dimer: a review
- PMID: 21686170
- PMCID: PMC3116176
- DOI: 10.3390/ijms12053072
Antioxidant properties of aminoethylcysteine ketimine decarboxylated dimer: a review
Abstract
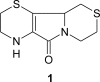
Aminoethylcysteine ketimine decarboxylated dimer is a natural sulfur-containing compound detected in human plasma and urine, in mammalian brain and in many common edible vegetables. Over the past decade many studies have been undertaken to identify its metabolic role. Attention has been focused on its antioxidant properties and on its reactivity against oxygen and nitrogen reactive species. These properties have been studied in different model systems starting from plasma lipoproteins to specific cellular lines. All these studies report that aminoethylcysteine ketimine decarboxylated dimer is able to interact both with reactive oxygen and nitrogen species (hydrogen peroxide, superoxide anion, hydroxyl radical, peroxynitrite and its derivatives). Its antioxidant activity is similar to that of Vitamin E while higher than other hydrophilic antioxidants, such as trolox and N-acetylcysteine.
Keywords: aminoethylcysteine ketimine decarboxylated dimer; reactive nitrogen species; reactive oxygen species; sulfur-containing antioxidants.
Figures

References
-
- Davies K. Oxidative stress: The paradox of aerobic life. Biochem. Soc. Symp. 1995;61:1–31. - PubMed
-
- Sies H. Oxidative stress: Oxidants and antioxidants. Exp. Physiol. 1997;82:291–295. - PubMed
-
- Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: Systematic review and meta-analysis. JAMA. 2007;297:842–857. - PubMed
-
- Matill HA. Antioxidants. Ann. Rev. Biochem. 1947;16:177–192. - PubMed
-
- Vertuani S, Angusti A, Manfredini S. The antioxidants and pro-antioxidants network: An overview. Curr. Pharm. Des. 2004;10:1677–1694. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

