CONSORT 2010 Statement: updated guidelines for reporting parallel group randomized trials
- PMID: 21686296
- PMCID: PMC3116666
CONSORT 2010 Statement: updated guidelines for reporting parallel group randomized trials
Conflict of interest statement
Competing interests: Uniform disclosure of potential conflicts of interest: all authors have completed the
Figures
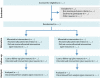


References
-
- Jüni P, Altman D G, Egger M. Systematic reviews in health care: Assessing the quality of controlled clinical trials. BMJ. 2001;323(7303):42–46. http://bmj.com/cgi/pmidlookup?view=long&pmid=11440947. - PMC - PubMed
-
- Dwan Kerry, Altman Douglas G, Arnaiz Juan A, Bloom Jill, Chan An-Wen, Cronin Eugenia, Decullier Evelyne, Easterbrook Philippa J, von Elm Erik, Gamble Carrol, Ghersi Davina, Ioannidis John P A, Simes John, Williamson Paula R. Systematic review of the empirical evidence of study publication bias and outcome reporting bias. PLoS One. 2008 Aug 28;3(8):e3081. doi: 10.1371/journal.pone.0003081. http://dx.plos.org/10.1371/journal.pone.0003081. - DOI - DOI - PMC - PubMed
-
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, Pitkin R, Rennie D, Schulz K F, Simel D, Stroup D F. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA. 1996;276(8):637–639. - PubMed
LinkOut - more resources
Full Text Sources
Medical
