Intravenous immunoglobulin for the treatment of chronic inflammatory demyelinating polyradiculoneuropathy: a systematic review and meta-analysis
- PMID: 21687335
- PMCID: PMC3090105
Intravenous immunoglobulin for the treatment of chronic inflammatory demyelinating polyradiculoneuropathy: a systematic review and meta-analysis
Abstract
Background: Chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) is an acquired immune-mediated inflammatory disorder that targets the myelin sheaths of the peripheral nervous system. Intravenous immunoglobulin (IVIg) is a blood product containing immunoglobulin G pooled from many human donors. In fall 2008, CIDP became an approved indication for IVIg in the United States and Canada.
Objective: To evaluate the clinical effectiveness and safety of IVIg for the treatment of CIDP through a systematic review of published randomized controlled trials.
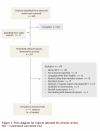
Methods: We searched the MEDLINE (1996-2009, including in-process and other non-indexed citations), Embase (1996-2009) and other databases through the Ovid interface. We applied a methodological filter to limit retrieval to controlled clinical trials, meta-analyses and systematic reviews, and health technology assessments. Retrieval was limited to studies involving humans, and no language restrictions were employed. We pooled extracted data to estimate the effect size of IVIg treatment based on the random-effects model.
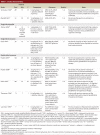
Results: We identified 9 unique randomized controlled trials. Of these, 3 compared IVIg therapy with an active comparator (plasma exchange, plasma exchange using extracorporeal immunoadsorption, oral prednisolone, respectively); the other 6 trials had placebo controls. No incremental benefit was seen in terms of primary outcomes for comparisons of IVIg therapy and an active comparator. Data from 4 of the 6 placebo-controlled trials were included in a meta-analysis. A significant improvement in disability (i.e., reduction in disability score) was found, with a standardized mean difference of 0.65 (95% confidence interval [CI] 0.23 to 1.08) in favour of IVIg. A pooled analysis of the proportion of patients with a response to treatment, as defined by the investigators of each of the trials, resulted in a risk ratio of 2.74 (95% CI 1.80 to 4.15) favouring IVIg.
Interpretation: IVIg therapy was statistically superior to placebo in reducing disability and impairment among patients with CIDP. The effectiveness of IVIg was similar to that of the alternative treatment strategies of plasma exchange and oral prednisolone.
Conflict of interest statement
Competing interests: Kathryn Gaebel has received funds from Abbott Laboratories Ltd. for a speaking engagement. Gord Blackhouse received funding from Eli Lilly Canada Inc. and GlaxoSmithKline Inc. for consulting. Colin Chalk received funding from Talecris Biotherapeutics Ltd., and he is the primary investigator in a multi-centre study funded by Baxter Canada. Mitchell Levine received funding from AstraZeneca Canada Inc., Eli Lilly Canada Inc., Pfizer Canada Inc. and Merck Frosst Canada Inc. He has acted as an expert witness for Novartis Pharmaceuticals Canada Inc. and for Wyeth Pharmaceuticals. He has also chaired the Expert Drug Committee for Health Canada. Ron Goeree was advisory board member to Janssen-Ortho Inc. and Hoffmann-La Roche Ltd. He has been a consultant for Eli Lilly Canada Inc.
Figures


References
-
- McLeod J G, Pollard J D, Macaskill P, Mohamed A, Spring P, Khurana V. Prevalence of chronic inflammatory demyelinating polyneuropathy in New South Wales, Australia. Ann Neurol. 1999;46(6):910–913. - PubMed
-
- Lunn M P, Manji H, Choudhary P P, Hughes R A, Thomas P K. Chronic inflammatory demyelinating polyradiculoneuropathy: a prevalence study in south east England. J Neurol Neurosurg Psychiatry. 1999;66(5):677–680. http://jnnp.bmj.com/cgi/pmidlookup?view=long&pmid=10209187. - PMC - PubMed
LinkOut - more resources
Full Text Sources
