Molecular Characterization of Exploitation of the Polyubiquitination and Farnesylation Machineries of Dictyostelium Discoideum by the AnkB F-Box Effector of Legionella Pneumophila
- PMID: 21687415
- PMCID: PMC3109286
- DOI: 10.3389/fmicb.2011.00023
Molecular Characterization of Exploitation of the Polyubiquitination and Farnesylation Machineries of Dictyostelium Discoideum by the AnkB F-Box Effector of Legionella Pneumophila
Abstract
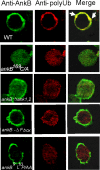
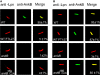
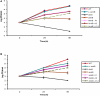
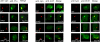
The Dot/Icm-translocated Ankyrin B (AnkB) F-box effector of Legionella pneumophila is essential for intra-vacuolar proliferation and functions as a platform for the docking of polyubiquitinated proteins to the Legionella-containing vacuole (LCV) within macrophages and ameba. Here we show that ectopically expressed AnkB in Dictyostelium discoideum is targeted to the plasma membrane where it recruits polyubiquitinated proteins and it trans-rescues the intracellular growth defect of the ankB null mutant, which has never been demonstrated for any effector in ameba. Using co-immunoprecipitation and bimolecular fluorescence complementation we show specific interaction of Skp1 of D. discoideum with the F-box domain of AnkB, which has never been demonstrated in ameba. We show that anchoring of AnkB to the cytosolic face of the LCV membrane in D. discoideum is mediated by the host farnesylation of the C-terminal eukaryotic CaaX motif of AnkB and is independent of the F-box and the two ANK domains, which has never been demonstrated in ameba. Importantly, the three host farnesylation enzymes farnesyl transferase, RCE-1, and isoprenyl cysteine carboxyl methyl transferase of D. discoideum are recruited to the LCV in a Dot/Icm-dependent manner, which has never been demonstrated in ameba. We conclude that the polyubiquitination and farnesylation enzymatic machineries of D. discoideum are recruited to the LCV in a Dot/Icm-dependent manner and the AnkB effector exploits the two evolutionarily conserved eukaryotic machineries to proliferate within ameba, similar to mammalian cells. We propose that L. pneumophila has acquired ankB through inter-kingdom horizontal gene transfer from primitive eukaryotes, which facilitated proliferation of L. pneumophila within human cells and the emergence of Legionnaires' disease.
Keywords: SCF1; Skp1; dot/Icm; farnesyl; prenylation.
Figures




References
-
- Amer A. O. (2010). Modulation of caspases and their non-apoptotic functions by Legionella pneumophila. Cell. Microbiol. 12, 140–147 - PubMed
-
- Berger K. H., Isberg R. R. (1993). Two distinct defects in intracellular growth complemented by a single genetic locus in Legionella pneumophila. Mol. Microbiol. 7, 7–19 - PubMed
-
- Bergo M. O., Leung G. K., Ambroziak P., Otto J. C., Casey P. J., Young S. G. (2000). Targeted inactivation of the isoprenylcysteine carboxyl methyltransferase gene causes mislocalization of K-Ras in mammalian cells. J. Biol. Chem. 275, 17605–17610 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

