ELAN: a software package for analysis and visualization of MEG, EEG, and LFP signals
- PMID: 21687568
- PMCID: PMC3113286
- DOI: 10.1155/2011/158970
ELAN: a software package for analysis and visualization of MEG, EEG, and LFP signals
Abstract
The recent surge in computational power has led to extensive methodological developments and advanced signal processing techniques that play a pivotal role in neuroscience. In particular, the field of brain signal analysis has witnessed a strong trend towards multidimensional analysis of large data sets, for example, single-trial time-frequency analysis of high spatiotemporal resolution recordings. Here, we describe the freely available ELAN software package which provides a wide range of signal analysis tools for electrophysiological data including scalp electroencephalography (EEG), magnetoencephalography (MEG), intracranial EEG, and local field potentials (LFPs). The ELAN toolbox is based on 25 years of methodological developments at the Brain Dynamics and Cognition Laboratory in Lyon and was used in many papers including the very first studies of time-frequency analysis of EEG data exploring evoked and induced oscillatory activities in humans. This paper provides an overview of the concepts and functionalities of ELAN, highlights its specificities, and describes its complementarity and interoperability with other toolboxes.
Figures
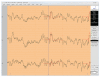

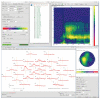


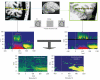
References
-
- Ravel N, Chabaud P, Martin C, et al. Olfactory learning modifies the expression of odour-induced oscillatory responses in the gamma (60–90 Hz) and beta (15–40 Hz) bands in the rat olfactory bulb. European Journal of Neuroscience. 2003;17(2):350–358. - PubMed
-
- Rols G, Tallon-Baudry C, Girard P, Bertrand O, Bullier J. Cortical mapping of gamma oscillations in areas V1 and V4 of the macaque monkey. Visual Neuroscience. 2001;18(4):527–540. - PubMed
-
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods. 2004;134(1):9–21. - PubMed
-
- Ruby P, Caclin A, Boulet S, Delpuech C, Morlet D. Odd sound processing in the sleeping brain. Journal of Cognitive Neuroscience. 2008;20(2):296–311. - PubMed
-
- Caclin A, McAdams S, Smith BK, Giard MH. Interactive processing of timbre dimensions: an exploration with event-related potentials. Journal of Cognitive Neuroscience. 2008;20(1):49–64. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

