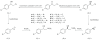Chiral Pharmaceutical Intermediaries Obtained by Reduction of 2-Halo-1-(4-substituted phenyl)-ethanones Mediated by Geotrichum candidum CCT 1205 and Rhodotorula glutinis CCT 2182
- PMID: 21687613
- PMCID: PMC3113109
- DOI: 10.4061/2011/976368
Chiral Pharmaceutical Intermediaries Obtained by Reduction of 2-Halo-1-(4-substituted phenyl)-ethanones Mediated by Geotrichum candidum CCT 1205 and Rhodotorula glutinis CCT 2182
Abstract
Enantioselective reductions of p-R(1)-C(6)H(4)C(O)CH(2)R(2) (R(1) = Cl, Br, CH(3), OCH(3), NO(2) and R(2) = Br, Cl) mediated by Geotrichum candidum CCT 1205 and Rhodotorula glutinis CCT 2182 afforded the corresponding halohydrins with complementary R and S configurations, respectively, in excellent yield and enantiomeric excesses. The obtained (R)- or (S)-halohydrins are important building blocks in chemical and pharmaceutical industries.
Figures
Similar articles
-
Highly enantioselective production of (R)-halohydrins with whole cells of Rhodotorula rubra KCh 82 culture.Int J Mol Sci. 2014 Dec 4;15(12):22392-404. doi: 10.3390/ijms151222392. Int J Mol Sci. 2014. PMID: 25486054 Free PMC article.
-
Highly enantiomeric reduction of acetophenone and its derivatives by locally isolated Rhodotorula glutinis.Chirality. 2010 Oct;22(9):849-54. doi: 10.1002/chir.20846. Chirality. 2010. PMID: 20803750
-
Microbial/enzymatic synthesis of chiral drug intermediates.Adv Appl Microbiol. 2000;47:33-78. doi: 10.1016/s0065-2164(00)47001-2. Adv Appl Microbiol. 2000. PMID: 12876794 Review.
-
Biocatalytic production of Ɛ-caprolactone using Geotrichum candidum cells immobilized on functionalized silica.Appl Microbiol Biotechnol. 2020 Oct;104(20):8887-8895. doi: 10.1007/s00253-020-10875-7. Epub 2020 Sep 9. Appl Microbiol Biotechnol. 2020. PMID: 32902680
-
Safety assessment of dairy microorganisms: Geotrichum candidum.Int J Food Microbiol. 2008 Sep 1;126(3):327-32. doi: 10.1016/j.ijfoodmicro.2007.08.021. Epub 2007 Aug 22. Int J Food Microbiol. 2008. PMID: 17869364 Review.
Cited by
-
Highly enantioselective production of (R)-halohydrins with whole cells of Rhodotorula rubra KCh 82 culture.Int J Mol Sci. 2014 Dec 4;15(12):22392-404. doi: 10.3390/ijms151222392. Int J Mol Sci. 2014. PMID: 25486054 Free PMC article.
References
-
- Brenelli ECS, Carvalho M, Okubo MT, et al. Enantioselective synthesis of (R)-(-)-1-phenylethanolamines using Baker’s yeast reduction of some α-substituted methyl phenyl ketones. Indian Journal of Chemistry B. 1992;31:821–823.
-
- Barbieri C, Bossi L, D’Arrigo P, Fantoni GP, Servi S. Bioreduction of aromatic ketones: preparation of chiral benzyl alcohols in both enantiomeric forms. Journal of Molecular Catalysis B. 2001;11(4–6):415–421.
-
- Patel RN. Biocatalysis: synthesis of chiral intermediates for drugs. Current Opinion in Drug Discovery and Development. 2006;9(6):741–764. - PubMed
-
- Breuer M, Ditrich K, Habicher T, et al. Industrial methods for the production of optically active intermediates. Angewandte Chemie International Edition. 2004;43(7):788–824. - PubMed
-
- De Carvalho M, Okamoto MT, Moran PJS, Rodrigues JAR. Baker’s yeast reduction of α-haloacetophenones. Tetrahedron. 1991;47(12-13):2073–2080.
LinkOut - more resources
Full Text Sources
Other Literature Sources