The nucleosome (histone-DNA complex) is the TLR9-specific immunostimulatory component of Plasmodium falciparum that activates DCs
- PMID: 21687712
- PMCID: PMC3110622
- DOI: 10.1371/journal.pone.0020398
The nucleosome (histone-DNA complex) is the TLR9-specific immunostimulatory component of Plasmodium falciparum that activates DCs
Abstract
The systemic clinical symptoms of Plasmodium falciparum infection such as fever and chills correspond to the proinflammatory cytokines produced in response to the parasite components released during the synchronized rupture of schizonts. We recently demonstrated that, among the schizont-released products, merozoites are the predominant components that activate dendritic cells (DCs) by TLR9-specific recognition to induce the maturation of cells and to produce proinflammatory cytokines. We also demonstrated that DNA is the active constituent and that formation of a DNA-protein complex is essential for the entry of parasite DNA into cells for recognition by TLR9. However, the nature of endogenous protein-DNA complex in the parasite is not known. In this study, we show that parasite nucleosome constitute the major protein-DNA complex involved in the activation of DCs by parasite nuclear material. The parasite components were fractionated into the nuclear and non-nuclear materials. The nuclear material was further fractionated into chromatin and the proteins loosely bound to chromatin. Polynucleosomes and oligonucleosomes were prepared from the chromatin. These were tested for their ability to activate DCs obtained by the FLT3 ligand differentiation of bone marrow cells from the wild type, and TLR2(-/-), TLR9(-/-) and MyD88(-/-) mice. DCs stimulated with the nuclear material and polynucleosomes as well as mono- and oligonucleosomes efficiently induced the production of proinflammatory cytokines in a TLR9-dependent manner, demonstrating that nucleosomes (histone-DNA complex) represent the major TLR9-specific DC-immunostimulatory component of the malaria parasite nuclear material. Thus, our data provide a significant insight into the activation of DCs by malaria parasites and have important implications for malaria vaccine development.
Conflict of interest statement
Figures


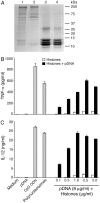
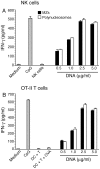
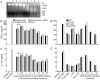
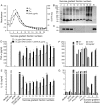
 ), and DNA contents (O) of the fractions were measured as described in “Materials and Methods”, and plotted. Panel B: SDS-PAGE analysis. The sucrose gradient fractions were dialyzed; aliquots corresponding to 20 µg of proteins were analyzed by SDS-PAGE using 15% gels. Sucrose gradient fraction numbers are indicated at the top. The mobility of molecular weight marker proteins is indicated to the left. Lane S, a mixture of standard H1, H2A, H2B, H3 and H4 histones. Panel C: Western blotting of sucrose gradient fractions using anti-H3 histone polyclonal antibodies. Lane descriptions are same as that in panel B. Panels D and E: TNF-α and IL-12 produced by WT FL-DCs stimulated with the indicated sucrose gradient fractions having 2.5 µg/ml of DNA content or with fractions having 2.5 µg/ml of DNA content to which the purified parasite genomic DNA (pDNA, 8 µg/ml) was added. Cells stimulated with parasite genomic DNA were used as controls. Panels F and G: TNF-α and IL-12 produced by FL-DCs from WT, TLR2−/−, TLR9−/− and MyD88−/− stimulated with sucrose gradient fractions. DCs stimulated with Pam3CSK4, Poly I∶C, LPS, CpG ODN or parasite genomic DNA were used as a control. The analysis was repeated three times each time performed in duplicates, and results of a representative are shown. Error bars represent mean values ± SEM. * p<0.05; ** p<0.01 comparison between sucrose gradient fractions with and without added parasite DNA.
), and DNA contents (O) of the fractions were measured as described in “Materials and Methods”, and plotted. Panel B: SDS-PAGE analysis. The sucrose gradient fractions were dialyzed; aliquots corresponding to 20 µg of proteins were analyzed by SDS-PAGE using 15% gels. Sucrose gradient fraction numbers are indicated at the top. The mobility of molecular weight marker proteins is indicated to the left. Lane S, a mixture of standard H1, H2A, H2B, H3 and H4 histones. Panel C: Western blotting of sucrose gradient fractions using anti-H3 histone polyclonal antibodies. Lane descriptions are same as that in panel B. Panels D and E: TNF-α and IL-12 produced by WT FL-DCs stimulated with the indicated sucrose gradient fractions having 2.5 µg/ml of DNA content or with fractions having 2.5 µg/ml of DNA content to which the purified parasite genomic DNA (pDNA, 8 µg/ml) was added. Cells stimulated with parasite genomic DNA were used as controls. Panels F and G: TNF-α and IL-12 produced by FL-DCs from WT, TLR2−/−, TLR9−/− and MyD88−/− stimulated with sucrose gradient fractions. DCs stimulated with Pam3CSK4, Poly I∶C, LPS, CpG ODN or parasite genomic DNA were used as a control. The analysis was repeated three times each time performed in duplicates, and results of a representative are shown. Error bars represent mean values ± SEM. * p<0.05; ** p<0.01 comparison between sucrose gradient fractions with and without added parasite DNA.Similar articles
-
Protein-DNA complex is the exclusive malaria parasite component that activates dendritic cells and triggers innate immune responses.J Immunol. 2010 Apr 15;184(8):4338-48. doi: 10.4049/jimmunol.0903824. Epub 2010 Mar 15. J Immunol. 2010. PMID: 20231693 Free PMC article.
-
Malaria blood stage parasites activate human plasmacytoid dendritic cells and murine dendritic cells through a Toll-like receptor 9-dependent pathway.J Immunol. 2004 Apr 15;172(8):4926-33. doi: 10.4049/jimmunol.172.8.4926. J Immunol. 2004. PMID: 15067072
-
TLR9-dependent activation of dendritic cells by DNA from Leishmania major favors Th1 cell development and the resolution of lesions.J Immunol. 2009 Feb 1;182(3):1386-96. doi: 10.4049/jimmunol.182.3.1386. J Immunol. 2009. PMID: 19155485
-
Antigen presentation and dendritic cell biology in malaria.Parasite Immunol. 2006 Jan-Feb;28(1-2):5-14. doi: 10.1111/j.1365-3024.2006.00772.x. Parasite Immunol. 2006. PMID: 16438671 Review.
-
Parasite Recognition and Signaling Mechanisms in Innate Immune Responses to Malaria.Front Immunol. 2018 Dec 19;9:3006. doi: 10.3389/fimmu.2018.03006. eCollection 2018. Front Immunol. 2018. PMID: 30619355 Free PMC article. Review.
Cited by
-
Histones from dying renal cells aggravate kidney injury via TLR2 and TLR4.J Am Soc Nephrol. 2012 Aug;23(8):1375-88. doi: 10.1681/ASN.2011111077. Epub 2012 Jun 7. J Am Soc Nephrol. 2012. PMID: 22677551 Free PMC article.
-
TLR9 and MyD88 are crucial for the development of protective immunity to malaria.J Immunol. 2012 May 15;188(10):5073-85. doi: 10.4049/jimmunol.1102143. Epub 2012 Apr 18. J Immunol. 2012. PMID: 22516959 Free PMC article.
-
Innate immunity to malaria: The good, the bad and the unknown.Front Immunol. 2022 Aug 19;13:914598. doi: 10.3389/fimmu.2022.914598. eCollection 2022. Front Immunol. 2022. PMID: 36059493 Free PMC article. Review.
-
Steatotic Donor Transplant Livers: Preservation Strategies to Mitigate against Ischaemia-Reperfusion Injury.Int J Mol Sci. 2024 Apr 24;25(9):4648. doi: 10.3390/ijms25094648. Int J Mol Sci. 2024. PMID: 38731866 Free PMC article. Review.
-
A surprising role for uric acid: the inflammatory malaria response.Curr Rheumatol Rep. 2014 Feb;16(2):401. doi: 10.1007/s11926-013-0401-8. Curr Rheumatol Rep. 2014. PMID: 24390755 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

