Temporal analysis of the honey bee microbiome reveals four novel viruses and seasonal prevalence of known viruses, Nosema, and Crithidia
- PMID: 21687739
- PMCID: PMC3110205
- DOI: 10.1371/journal.pone.0020656
Temporal analysis of the honey bee microbiome reveals four novel viruses and seasonal prevalence of known viruses, Nosema, and Crithidia
Abstract
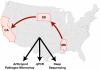
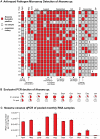
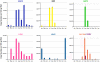
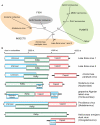
Honey bees (Apis mellifera) play a critical role in global food production as pollinators of numerous crops. Recently, honey bee populations in the United States, Canada, and Europe have suffered an unexplained increase in annual losses due to a phenomenon known as Colony Collapse Disorder (CCD). Epidemiological analysis of CCD is confounded by a relative dearth of bee pathogen field studies. To identify what constitutes an abnormal pathophysiological condition in a honey bee colony, it is critical to have characterized the spectrum of exogenous infectious agents in healthy hives over time. We conducted a prospective study of a large scale migratory bee keeping operation using high-frequency sampling paired with comprehensive molecular detection methods, including a custom microarray, qPCR, and ultra deep sequencing. We established seasonal incidence and abundance of known viruses, Nosema sp., Crithidia mellificae, and bacteria. Ultra deep sequence analysis further identified four novel RNA viruses, two of which were the most abundant observed components of the honey bee microbiome (∼10(11) viruses per honey bee). Our results demonstrate episodic viral incidence and distinct pathogen patterns between summer and winter time-points. Peak infection of common honey bee viruses and Nosema occurred in the summer, whereas levels of the trypanosomatid Crithidia mellificae and Lake Sinai virus 2, a novel virus, peaked in January.
Conflict of interest statement
Figures

References
-
- Pennisi E. Genetics - Honey bee genome illuminates insect evolution and social behavior. Science. 2006;314:578–579. - PubMed
-
- Winston ML. 1987. The biology of the Honey Bee: First Harvard Press.
-
- Johnson R. 2010. Honey Bee Colony Collapse Disorder.
-
- USDA-ARS Questions and Answers: Colony Collapse Disorder
-
- Morse RA, Calderone NW. The Value of Honey Bees as Pollinators of U.S. Crops in 2000. Gleanings in Bee Culture Supple. 2000:1–15.
Publication types
MeSH terms
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

