Role of phospholipases in fungal fitness, pathogenicity, and drug development - lessons from cryptococcus neoformans
- PMID: 21687772
- PMCID: PMC3109512
- DOI: 10.3389/fmicb.2010.00125
Role of phospholipases in fungal fitness, pathogenicity, and drug development - lessons from cryptococcus neoformans
Abstract
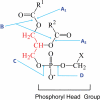
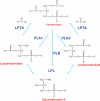
Many pathogenic microbes, including many fungi, produce phospholipases which facilitate survival of the pathogen in vivo, invasion and dissemination throughout the host, expression of virulence traits and evasion of host immune defense mechanisms. These phospholipases are either secreted or produced intracellularly and act by physically disrupting host membranes, and/or by affecting fungal cell signaling and production of immunomodulatory effectors. Many of the secreted phospholipases acquire a glycosylphosphatidylinositol sorting motif to facilitate membrane and/or cell wall association and secretion. This review focuses primarily on the role of two members of the phospholipase enzyme family, phospholipase B (Plb) and phosphatidylinositol (PI)-specific phospholipase C (PI-C/Plc), in fungal pathogenesis and in particular, what has been learnt about their function from studies performed in the model pathogenic yeast, Cryptococcus neoformans. These studies have revealed how Plb has adapted to become an important part of the virulence repertoire of pathogenic fungi and how its secretion is regulated. They have also provided valuable insight into how the intracellular enzyme, Plc1, contributes to fungal fitness and pathogenicity - via a putative role in signal transduction pathways that regulate the production of stress-protecting pigments, polysaccharide capsule, cell wall integrity, and adaptation to growth at host temperature. Finally, this review will address the role fungal phospholipases have played in the development of a new class of antifungal drugs, which mimic their phospholipid substrates.
Keywords: Cryptococcus neoformans; PI-PLC/Plc; drug development; pathogenicity; phosphatidylinositol-specific phospholipase C; phospholipase B; secretion; signaling.
Figures

References
-
- Alspaugh J. A., Pukkila-Worley R., Harashima T., Cavallo L. M., Funnell D., Cox G. M., Perfect J. R., Kronstad J. W., Heitman J. (2002). Adenylyl cyclase functions downstream of the Galpha protein Gpa1 and controls mating and pathogenicity of Cryptococcus neoformans. Eukaryotic Cell 1, 75–84 10.1101/gad.11.23.3206 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

