Individualised therapy of angiotensin converting enzyme (ACE) inhibitors in stable coronary artery disease: overview of the primary results of the PERindopril GENEtic association (PERGENE) study
- PMID: 21688035
- PMCID: PMC3247631
- DOI: 10.1007/s12471-011-0173-6
Individualised therapy of angiotensin converting enzyme (ACE) inhibitors in stable coronary artery disease: overview of the primary results of the PERindopril GENEtic association (PERGENE) study
Abstract
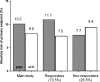
In patients with stable coronary artery disease (CAD) without overt heart failure, ACE inhibitors are among the most commonly used drugs as these agents have been proven effective in reducing the risk of cardiovascular events. Considerable individual variations in the blood pressure response to ACE inhibitors are observed and as such heterogeneity in clinical treatment effect would be likely as well. Assessing the consistency of treatment benefit is essential for the rational and cost-effective prescription of ACE inhibitors. Information on heterogeneities in treatment effect between subgroups of patients could be used to develop an evidence-based guidance for the installation of ACE-inhibitor therapy. Obviously, therapy should only be applied in those patients who most likely will benefit. Attempts to develop such treatment guidance by using clinical characteristics have been unsuccessful. No heterogeneity in risk reduction by ACE inhibitors has been observed in relation to relevant clinical characteristics. A new approach to such 'guided-therapy' could be to integrate more patient-specific characteristics such as the patients' genetic information. If proven feasible, pharmacogenetic profiling could optimise patients' benefit of treatment and reduce unnecessary treatment of patients. Cardiovascular pharmacogenetic research of ACE inhibitors in coronary artery disease patients is in a formative stage and studies are limited. The PERGENE study is a large pharmacogenetic substudy of the EUROPA trial, aimed to assess the achievability of pharmacogenetic profiling. We provide an overview of the main results of the PERGENE study in terms of the genetic determinants of treatment benefit and blood pressure response. The main results of the PERGENE study show a pharmacogenetic profile related to the treatment benefit of perindopril identifying responders and non-responders to treatment.
Figures





References
-
- Pellacani A, Brunner HR, Nussberger J. Plasma kinins increase after angiotensin enzyme inhibition in human subjects. Clin Sci (Lond) 1994;87:567–74. - PubMed
-
- Pfeffer MA, Braunwald E, Moye LA, et al. SAVE investigators. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction and myocardial infarction: results of the survival and ventricular enlargement trial. N Engl J Med. 1992;327:669–77. doi: 10.1056/NEJM199209033271001. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

