Primary neural degeneration in the Guinea pig cochlea after reversible noise-induced threshold shift
- PMID: 21688060
- PMCID: PMC3173555
- DOI: 10.1007/s10162-011-0277-0
Primary neural degeneration in the Guinea pig cochlea after reversible noise-induced threshold shift
Abstract
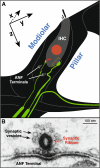
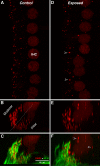
Recent work in mouse showed that acoustic overexposure can produce a rapid and irreversible loss of cochlear nerve peripheral terminals on inner hair cells (IHCs) and a slow degeneration of spiral ganglion cells, despite full recovery of cochlear thresholds and no loss of inner or outer hair cells (Kujawa and Liberman, J Neurosci 29:14077-14085, 2009). This contrasts with earlier ultrastructural work in guinea pig suggesting that acute noise-induced neural degeneration is followed by full regeneration of cochlear nerve terminals in the IHC area (Puel et al., Neuroreport 9:2109-2114, 1998; Pujol and Puel, Ann N Y Acad Sci 884:249-254, 1999). Here, we show that the same patterns of primary neural degeneration reported for mouse are also seen in the noise-exposed guinea pig, when IHC synapses and cochlear nerve terminals are counted 1 week post-exposure in confocal images from immunostained whole mounts and that the same slow degeneration of spiral ganglion cells occurs despite no loss of IHCs and apparent recovery of cochlear thresholds. The data cast doubt on prior claims that there is significant neural regeneration and synaptogenesis in the adult cochlea and suggest that denervation of the inner hair cell is an important sequela of "reversible" noise-induced hearing loss, which likely applies to the human ear as well.
Figures


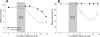


References
-
- Costalupes JA, Young ED, Gibson DJ. Effects of continuous noise backgrounds on rate response of auditory nerve fibers in cat. J Neurophysiol. 1984;51:1326–1344. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

