Isoform-specific dephosphorylation of dynamin1 by calcineurin couples neurotrophin receptor endocytosis to axonal growth
- PMID: 21689596
- PMCID: PMC3119854
- DOI: 10.1016/j.neuron.2011.04.025
Isoform-specific dephosphorylation of dynamin1 by calcineurin couples neurotrophin receptor endocytosis to axonal growth
Abstract
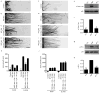
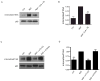
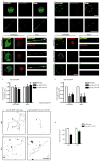
Endocytic events are critical for neuronal survival in response to target-derived neurotrophic cues, but whether local axon growth is mediated by endocytosis-dependent signaling mechanisms remains unclear. Here, we report that Nerve Growth Factor (NGF) promotes endocytosis of its TrkA receptors and axon growth by calcineurin-mediated dephosphorylation of the endocytic GTPase dynamin1. Conditional deletion of calcineurin in sympathetic neurons disrupts NGF-dependent innervation of peripheral target tissues. Calcineurin signaling is required locally in sympathetic axons to support NGF-mediated growth in a manner independent of transcription. We show that calcineurin associates with dynamin1 via a PxIxIT interaction motif found only in specific dynamin1 splice variants. PxIxIT-containing dynamin1 isoforms colocalize with surface TrkA receptors, and their phosphoregulation is selectively required for NGF-dependent TrkA internalization and axon growth in sympathetic neurons. Thus, NGF-dependent phosphoregulation of dynamin1 is a critical event coordinating neurotrophin receptor endocytosis and axonal growth.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures





References
-
- Aramburu J, Garcia-Cozar F, Raghavan A, Okamura H, Rao A, Hogan PG. Selective inhibition of NFAT activation by a peptide spanning the calcineurin targeting site of NFAT. Mol Cell. 1998;1:627–637. - PubMed
-
- Aramburu J, Yaffe MB, Lopez-Rodriguez C, Cantley LC, Hogan PG, Rao A. Affinity-driven peptide selection of an NFAT inhibitor more selective than cyclosporin A. Science. 1999;285:2129–2133. - PubMed
-
- Beattie EC, Carroll RC, Yu X, Morishita W, Yasuda H, von Zastrow M, Malenka RC. Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nat Neurosci. 2000;3:1291–1300. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

