Follistatin improves skeletal muscle healing after injury and disease through an interaction with muscle regeneration, angiogenesis, and fibrosis
- PMID: 21689628
- PMCID: PMC3157209
- DOI: 10.1016/j.ajpath.2011.04.008
Follistatin improves skeletal muscle healing after injury and disease through an interaction with muscle regeneration, angiogenesis, and fibrosis
Abstract
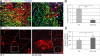
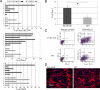
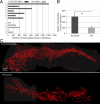
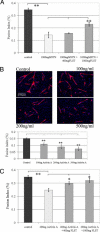
Recovery from skeletal muscle injury is often incomplete because of the formation of fibrosis and inadequate myofiber regeneration; therefore, injured muscle could benefit significantly from therapies that both stimulate muscle regeneration and inhibit fibrosis. To this end, we focused on blocking myostatin, a member of the transforming growth factor-β superfamily and a negative regulator of muscle regeneration, with the myostatin antagonist follistatin. In vivo, follistatin-overexpressing transgenic mice underwent significantly greater myofiber regeneration and had less fibrosis formation compared with wild-type mice after skeletal muscle injury. Follistatin's mode of action is likely due to its ability to block myostatin and enhance neovacularization. Furthermore, muscle progenitor cells isolated from follistatin-overexpressing mice were significantly superior to muscle progenitors isolated from wild-type mice at regenerating dystrophin-positive myofibers when transplanted into the skeletal muscle of dystrophic mdx/severe combined immunodeficiency mice. In vitro, follistatin stimulated myoblasts to express MyoD, Myf5, and myogenin, which are myogenic transcription factors that promote myogenic differentiation. Moreover, follistatin's ability to enhance muscle differentiation is at least partially due to its ability to block myostatin, activin A, and transforming growth factor-β1, all of which are negative regulators of muscle cell differentiation. The findings of this study suggest that follistatin is a promising agent for improving skeletal muscle healing after injury and muscle diseases, such as the muscular dystrophies.
Copyright © 2011 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Relationships between transforming growth factor-beta1, myostatin, and decorin: implications for skeletal muscle fibrosis.J Biol Chem. 2007 Aug 31;282(35):25852-63. doi: 10.1074/jbc.M704146200. Epub 2007 Jun 27. J Biol Chem. 2007. PMID: 17597062
-
Decorin gene transfer promotes muscle cell differentiation and muscle regeneration.Mol Ther. 2007 Sep;15(9):1616-22. doi: 10.1038/sj.mt.6300250. Epub 2007 Jul 3. Mol Ther. 2007. PMID: 17609657
-
Myostatin and MyoD family expression in skeletal muscle of IGF-1 knockout mice.Cell Biol Int. 2007 Oct;31(10):1274-9. doi: 10.1016/j.cellbi.2007.05.007. Epub 2007 May 21. Cell Biol Int. 2007. PMID: 17590360
-
Muscle regeneration through myostatin inhibition.Curr Opin Rheumatol. 2005 Nov;17(6):720-4. doi: 10.1097/01.bor.0000184163.61558.ca. Curr Opin Rheumatol. 2005. PMID: 16224249 Review.
-
Function of the myogenic regulatory factors Myf5, MyoD, Myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis.Semin Cell Dev Biol. 2017 Dec;72:19-32. doi: 10.1016/j.semcdb.2017.11.011. Epub 2017 Nov 15. Semin Cell Dev Biol. 2017. PMID: 29127046 Review.
Cited by
-
Impaired skeletal muscle regeneration in the absence of fibrosis during hibernation in 13-lined ground squirrels.PLoS One. 2012;7(11):e48884. doi: 10.1371/journal.pone.0048884. Epub 2012 Nov 14. PLoS One. 2012. PMID: 23155423 Free PMC article.
-
Current Progress and Challenges for Skeletal Muscle Differentiation from Human Pluripotent Stem Cells Using Transgene-Free Approaches.Stem Cells Int. 2018 Apr 11;2018:6241681. doi: 10.1155/2018/6241681. eCollection 2018. Stem Cells Int. 2018. PMID: 29760730 Free PMC article. Review.
-
Epigenetic modifications in muscle regeneration and progression of Duchenne muscular dystrophy.Clin Epigenetics. 2021 Jan 19;13(1):13. doi: 10.1186/s13148-021-01001-z. Clin Epigenetics. 2021. PMID: 33468200 Free PMC article. Review.
-
Genetic disruption of Smad7 impairs skeletal muscle growth and regeneration.J Physiol. 2015 Jun 1;593(11):2479-97. doi: 10.1113/JP270201. Epub 2015 May 15. J Physiol. 2015. PMID: 25854148 Free PMC article.
-
Follistatin-based ligand trap ACE-083 induces localized hypertrophy of skeletal muscle with functional improvement in models of neuromuscular disease.Sci Rep. 2019 Aug 6;9(1):11392. doi: 10.1038/s41598-019-47818-w. Sci Rep. 2019. PMID: 31388039 Free PMC article.
References
-
- Border W.A., Noble N.A. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994;331:1286–1292. - PubMed
-
- Lijnen P.J., Petrov V.V., Fagard R.H. Induction of cardiac fibrosis by transforming growth factor-beta(1) Mol Genet Metab. 2000;71:418–435. - PubMed
-
- Yamamoto T., Noble N.A., Miller D.E., Border W.A. Sustained expression of TGF-beta 1 underlies development of progressive kidney fibrosis. Kidney Int. 1994;45:916–927. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

