Neural circuit flexibility in a small sensorimotor system
- PMID: 21689926
- PMCID: PMC3177960
- DOI: 10.1016/j.conb.2011.05.019
Neural circuit flexibility in a small sensorimotor system
Abstract
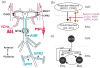
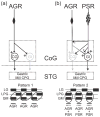
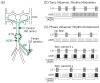
Neuronal circuits underlying rhythmic behaviors (central pattern generators: CPGs) can generate rhythmic motor output without sensory input. However, sensory input is pivotal for generating behaviorally relevant CPG output. Here we discuss recent work in the decapod crustacean stomatogastric nervous system (STNS) identifying cellular and synaptic mechanisms whereby sensory inputs select particular motor outputs from CPG circuits. This includes several examples in which sensory neurons regulate the impact of descending projection neurons on CPG circuits. This level of analysis is possible in the STNS due to the relatively unique access to identified circuit, projection, and sensory neurons. These studies are also revealing additional degrees of freedom in sensorimotor integration that underlie the extensive flexibility intrinsic to rhythmic motor systems.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures

References
-
- Marder E, Bucher D. Understanding circuit dynamics using the stomatogastric nervous system of lobsters and crabs. Annu Rev Physiol. 2007;69:291–316. - PubMed
-
- Briggman KL, Kristan WB. Multifunctional pattern-generating circuits. Annu Rev Neurosci. 2008;31:271–294. - PubMed
-
- Kiehn O, Dougherty KJ, Hägglund M, Borgius L, Talpalar A, Restrepo CE. Probing spinal circuits controlling walking in mammals. Biochem Biophys Res Commun. 2010;396:11–18. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

