Reproducible enrichment of extracellular heat shock proteins from blood serum using monomeric avidin
- PMID: 21689931
- PMCID: PMC3136036
- DOI: 10.1016/j.bmcl.2011.05.111
Reproducible enrichment of extracellular heat shock proteins from blood serum using monomeric avidin
Abstract
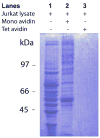
Extracellular heat shock proteins (eHsps) in blood circulation have been associated with various diseases, including cancer. However, the lack of methods to enrich eHsps from serum samples has hampered the characterization of eHsps. This Letter presents our serendipitous finding that the monomeric avidin resin can serve as an affinity resin to enrich eHsps from blood serum. Biochemical mechanism of this eHsp enrichment as well as implications in biomarker discovery is discussed.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures



Similar articles
-
Monolithic columns with immobilized monomeric avidin: preparation and application for affinity chromatography.Anal Bioanal Chem. 2012 Mar;402(7):2395-405. doi: 10.1007/s00216-011-5670-3. Epub 2012 Jan 20. Anal Bioanal Chem. 2012. PMID: 22262050
-
The dark-side of the outside: how extracellular heat shock proteins promote cancer.Cell Mol Life Sci. 2021 May;78(9):4069-4083. doi: 10.1007/s00018-021-03764-3. Epub 2021 Feb 5. Cell Mol Life Sci. 2021. PMID: 33544155 Free PMC article. Review.
-
Extracellular HSPs: The Potential Target for Human Disease Therapy.Molecules. 2022 Apr 6;27(7):2361. doi: 10.3390/molecules27072361. Molecules. 2022. PMID: 35408755 Free PMC article. Review.
-
First-trimester plasma extracellular heat shock proteins levels and risk of preeclampsia.J Cell Mol Med. 2023 May;27(9):1206-1213. doi: 10.1111/jcmm.17674. Epub 2023 Mar 31. J Cell Mol Med. 2023. PMID: 37002651 Free PMC article.
-
Extracellular Heat Shock Proteins as Therapeutic Targets and Biomarkers in Fibrosing Interstitial Lung Diseases.Int J Mol Sci. 2021 Aug 27;22(17):9316. doi: 10.3390/ijms22179316. Int J Mol Sci. 2021. PMID: 34502225 Free PMC article. Review.
References
-
- Hartl FU, Hayer-Hartl M. Science. 2002;295:1852. - PubMed
-
- Hendrick JP, Hartl FU. FASEB J. 1995;9:1559. - PubMed
-
- Hartl FU. Nature. 1996;381:571. - PubMed
-
- Beckmann RP, Mizzen LE, Welch WJ. Science. 1990;248:850. - PubMed
-
- Schmitt E, Gehrmann M, Brunet M, Multhoff G, Garrido C. J Leukoc Biol. 2007;81:15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

