Heparan sulfate proteoglycans
- PMID: 21690215
- PMCID: PMC3119907
- DOI: 10.1101/cshperspect.a004952
Heparan sulfate proteoglycans
Abstract
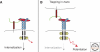
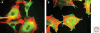
Heparan sulfate proteoglycans are found at the cell surface and in the extracellular matrix, where they interact with a plethora of ligands. Over the last decade, new insights have emerged regarding the mechanism and biological significance of these interactions. Here, we discuss changing views on the specificity of protein-heparan sulfate binding and the activity of HSPGs as receptors and coreceptors. Although few in number, heparan sulfate proteoglycans have profound effects at the cellular, tissue, and organismal level.
Figures



References
-
- Abrink M, Grujic M, Pejler G 2004. Serglycin is essential for maturation of mast cell secretory granule. J Biol Chem 279: 40897–40905 - PubMed
-
- Ai X, Kitazawa T, Do AT, Kusche-Gullberg M, Labosky PA, Emerson CP Jr 2007. SULF1 and SULF2 regulate heparan sulfate-mediated GDNF signaling for esophageal innervation. Development 134: 3327–3338 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
