Proteomics and pathway analyses of the milk fat globule in sheep naturally infected by Mycoplasma agalactiae provide indications of the in vivo response of the mammary epithelium to bacterial infection
- PMID: 21690237
- PMCID: PMC3165467
- DOI: 10.1128/IAI.00040-11
Proteomics and pathway analyses of the milk fat globule in sheep naturally infected by Mycoplasma agalactiae provide indications of the in vivo response of the mammary epithelium to bacterial infection
Abstract
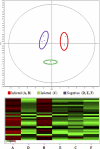
Milk fat globules (MFGs) are vesicles released in milk as fat droplets surrounded by the endoplasmic reticulum and apical cell membranes. During formation and apocrine secretion by lactocytes, various amounts of cytoplasmic crescents remain trapped within the released vesicle, making MFGs a natural sampling mechanism of the lactating cell contents. With the aim of investigating the events occurring in the mammary epithelium during bacterial infection, the MFG proteome was characterized by two-dimensional difference gel electrophoresis (2-D DIGE), SDS-PAGE followed by shotgun liquid chromatography-tandem mass spectrometry (GeLC-MS/MS), label-free quantification by the normalized spectral abundance factor (NSAF) approach, Western blotting, and pathway analysis, using sheep naturally infected by Mycoplasma agalactiae. A number of protein classes were found to increase in MFGs upon infection, including proteins involved in inflammation and host defense, cortical cytoskeleton proteins, heat shock proteins, and proteins related to oxidative stress. Conversely, a strikingly lower abundance was observed for proteins devoted to MFG metabolism and secretion. To our knowledge, this is the first report describing proteomic changes occurring in MFGs during sheep infectious mastitis. The results presented here offer new insights into the in vivo response of mammary epithelial cells to bacterial infection and open the way to the discovery of protein biomarkers for monitoring clinical and subclinical mastitis.
Figures
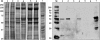





References
-
- Addis M. F., et al. 2009. Generation of high-quality protein extracts from formalin-fixed, paraffin-embedded tissues. Proteomics 9:3815–3823 - PubMed
-
- Addis M. F., Tanca A., Pagnozzi D., Rocca S., Uzzau S. 2009. 2-D PAGE and MS analysis of proteins from formalin-fixed, paraffin-embedded tissues. Proteomics 9:4329–4339 - PubMed
-
- Admyre C., et al. 2007. Exosomes with immune modulatory features are present in human breast milk. J. Immunol. 179:1969–1978 - PubMed
-
- Bergonier D., Berthelot X., Poumarat F. 1997. Contagious agalactia of small ruminants: current knowledge concerning epidemiology, diagnosis and control. Rev. Sci. Tech. 16:848–873 - PubMed
-
- Boehmer J. L., Bannerman D. D., Shefcheck K., Ward J. L. 2008. Proteomic analysis of differentially expressed proteins in bovine milk during experimentally induced Escherichia coli mastitis. J. Dairy Sci. 91:4206–4218 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

