An open-label crossover study to evaluate potential pharmacokinetic interactions between oral oseltamivir and intravenous zanamivir in healthy Thai adults
- PMID: 21690287
- PMCID: PMC3165358
- DOI: 10.1128/AAC.00159-11
An open-label crossover study to evaluate potential pharmacokinetic interactions between oral oseltamivir and intravenous zanamivir in healthy Thai adults
Abstract
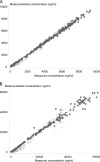
There is no parenteral formulation of the neuraminidase inhibitor oseltamivir, the most widely used anti-influenza virus drug. Oseltamivir resistance is an increasing problem. Zanamivir is effective against the most prevalent oseltamivir-resistant influenza viruses. A parenteral formulation of zanamivir is in development for the treatment of severe influenza. It is not known if there is any pharmacokinetic interaction between the two drugs. Sixteen healthy Thai adult volunteers were studied in an open-label, four-period, randomized two-sequence crossover pharmacokinetic study in which zanamivir was given by constant-rate infusion or slow intravenous injection either alone or together with oral oseltamivir. Plasma concentration profiles of oseltamivir, the active metabolite oseltamivir carboxylate, and zanamivir were measured by liquid chromatography-mass spectrometry-mass spectrometry. Both drugs were well tolerated alone and in combination. The maximum plasma concentrations and the areas under the plasma concentration-time curves (AUC) of oseltamivir and oseltamivir carboxylate were not significantly different when oseltamivir was given separately or together with zanamivir. Maximum plasma concentrations of zanamivir were 10% (95% confidence interval, 7 to 12%) higher when zanamivir was infused concurrently with oral oseltamivir than with infusions before or after oral oseltamivir. The plasma zanamivir total AUC was positively correlated with the total oseltamivir carboxylate AUC (Pearson's correlation coefficient [r(P)] = 0.720, P = 0.002, n = 16) but not with the oseltamivir AUC (r(p) = 0.121, n = 16). There is no clinically significant pharmacokinetic interaction between oseltamivir and zanamivir.
Figures

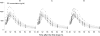
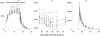

References
-
- Arya V., Carter W. W., Robertson S. M. 2010. The role of clinical pharmacology in supporting the emergency use authorization of an unapproved anti-influenza drug, peramivir. Clin. Pharmacol. Ther. 88:587–589 - PubMed
-
- Beigel J. H., et al. 2005. Current concepts–avian influenza A (H5N1) infection in humans. N. Engl. J. Med. 353:1374–1385 - PubMed
-
- Cass L. M. R., Efthymiopoulos C., Bye A. 1999. Pharmacokinetics of zanamivir after intravenous, oral, inhaled or intranasal administration to healthy volunteers. Clin. Pharmacokinet. 36(Suppl. 1):1–11 - PubMed
-
- Daniel M. J., Barnett J. M., Pearson B. A. 1999. The low potential for drug interactions with zanamivir. Clin. Pharmacokinet. 36(Suppl. 1):41–50 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

