Neurotrophin-mediated dendrite-to-nucleus signaling revealed by microfluidic compartmentalization of dendrites
- PMID: 21690335
- PMCID: PMC3131323
- DOI: 10.1073/pnas.1012401108
Neurotrophin-mediated dendrite-to-nucleus signaling revealed by microfluidic compartmentalization of dendrites
Abstract
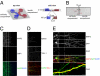
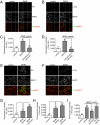
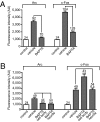
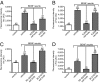
Signaling from dendritic synapses to the nucleus regulates important aspects of neuronal function, including synaptic plasticity. The neurotrophin brain-derived neurotrophic factor (BDNF) can induce long-lasting strengthening of synapses in vivo and this effect is dependent on transcription. However, the mechanism of signaling to the nucleus is not well understood. Here we describe a microfluidic culture device to investigate dendrite-to-nucleus signaling. Using these microfluidic devices, we demonstrate that BDNF can act directly on dendrites to elicit an anterograde signal that induces transcription of the immediate early genes, Arc and c-Fos. Induction of Arc is dependent on dendrite- and cell body-derived calcium, whereas induction of c-Fos is calcium-independent. In contrast to retrograde neurotrophin-mediated axon-to-nucleus signaling, which is MEK5-dependent, BDNF-mediated anterograde dendrite-to-nucleus signaling is dependent on MEK1/2. Intriguingly, the activity of TrkB, the BDNF receptor, is required in the cell body for the induction of Arc and c-Fos mediated by dendritically applied BDNF. These results are consistent with the involvement of a signaling endosome-like pathway that conveys BDNF signals from the dendrite to the nucleus.
Conflict of interest statement
Conflict of interest statement: N.L.J. is an inventor and patent holder of the microfluidic devices described in this article and is a cofounder of Xona Microfluidics LLC, which markets related microfluidic devices.
Figures
References
-
- Minichiello L. TrkB signalling pathways in LTP and learning. Nat Rev Neurosci. 2009;10:850–860. - PubMed
-
- Kang H, Welcher AA, Shelton D, Schuman EM. Neurotrophins and time: Different roles for TrkB signaling in hippocampal long-term potentiation. Neuron. 1997;19:653–664. - PubMed
-
- Minichiello L, et al. Essential role for TrkB receptors in hippocampus-mediated learning. Neuron. 1999;24:401–414. - PubMed
-
- Patterson SL, et al. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron. 1996;16:1137–1145. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

