Role of CB1 cannabinoid receptors on GABAergic neurons in brain aging
- PMID: 21690345
- PMCID: PMC3131310
- DOI: 10.1073/pnas.1016442108
Role of CB1 cannabinoid receptors on GABAergic neurons in brain aging
Abstract
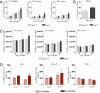
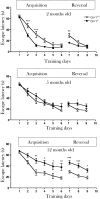
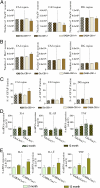
Brain aging is associated with cognitive decline that is accompanied by progressive neuroinflammatory changes. The endocannabinoid system (ECS) is involved in the regulation of glial activity and influences the progression of age-related learning and memory deficits. Mice lacking the Cnr1 gene (Cnr1(-/-)), which encodes the cannabinoid receptor 1 (CB1), showed an accelerated age-dependent deficit in spatial learning accompanied by a loss of principal neurons in the hippocampus. The age-dependent decrease in neuronal numbers in Cnr1(-/-) mice was not related to decreased neurogenesis or to epileptic seizures. However, enhanced neuroinflammation characterized by an increased density of astrocytes and activated microglia as well as an enhanced expression of the inflammatory cytokine IL-6 during aging was present in the hippocampus of Cnr1(-/-) mice. The ongoing process of pyramidal cell degeneration and neuroinflammation can exacerbate each other and both contribute to the cognitive deficits. Deletion of CB1 receptors from the forebrain GABAergic, but not from the glutamatergic neurons, led to a similar neuronal loss and increased neuroinflammation in the hippocampus as observed in animals lacking CB1 receptors in all cells. Our results suggest that CB1 receptor activity on hippocampal GABAergic neurons protects against age-dependent cognitive decline by reducing pyramidal cell degeneration and neuroinflammation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Murakami S, Murakami H. The effects of aging and oxidative stress on learning behavior in C. elegans. Neurobiol Aging. 2005;26:899–905. - PubMed
-
- Li SC, et al. Transformations in the couplings among intellectual abilities and constituent cognitive processes across the life span. Psychol Sci. 2004;15:155–163. - PubMed
-
- Kirkwood TB, Franceschi C. Is aging as complex as it would appear? New perspectives in aging research. Ann N Y Acad Sci. 1992;663:412–417. - PubMed
-
- Perry VH, Nicoll JA, Holmes C. Microglia in neurodegenerative disease. Nat Rev Neurol. 2010;6:193–201. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

