Functional motor recovery from brain ischemic insult by carbon nanotube-mediated siRNA silencing
- PMID: 21690348
- PMCID: PMC3131324
- DOI: 10.1073/pnas.1100930108
Functional motor recovery from brain ischemic insult by carbon nanotube-mediated siRNA silencing
Abstract
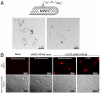
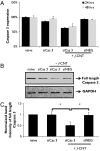
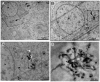
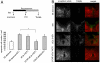
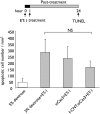
Stroke is the second cause of death worldwide with ischemic stroke accounting for 80% of all stroke insults. Caspase-3 activation contributes to brain tissue loss and downstream biochemical events that lead to programmed cell death after traumatic brain injury. Alleviation of symptoms following ischemic neuronal injury can be potentially achieved by either genetic disruption or pharmacological inhibition of caspases. Here, we studied whether silencing of Caspase-3 using carbon nanotube-mediated in vivo RNA interference (RNAi) could offer a therapeutic opportunity against stroke. Effective delivery of siRNA directly to the CNS has been shown to normalize phenotypes in animal models of several neurological diseases. It is shown here that peri-lesional stereotactic administration of a Caspase-3 siRNA (siCas 3) delivered by functionalized carbon nanotubes (f-CNT) reduced neurodegeneration and promoted functional preservation before and after focal ischemic damage of the rodent motor cortex using an endothelin-1 induced stroke model. These observations illustrate the opportunity offered by carbon nanotube-mediated siRNA delivery and gene silencing of neuronal tissue applicable to a variety of different neuropathological conditions where intervention at well localized brain foci may offer therapeutic and functional benefits.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Budihardjo I, Oliver H, Lutter M, Luo X, Wang X. Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol. 1999;15:269–290. - PubMed
-
- Slee EA, Adrain C, Martin SJ. Serial killers: ordering caspase activation events in apoptosis. Cell Death Differ. 1999;6:1067–1074. - PubMed
-
- Clark RS, et al. Caspase-3 mediated neuronal death after traumatic brain injury in rats. J Neurochem. 2000;74:740–753. - PubMed
-
- Chu Y, Miller JD, Heistad DD. Gene therapy for stroke: 2006 overview. Curr Hypertens Rep. 2007;9:19–24. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

