Measuring single-cell density
- PMID: 21690360
- PMCID: PMC3131325
- DOI: 10.1073/pnas.1104651108
Measuring single-cell density
Abstract
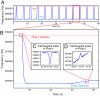
We have used a microfluidic mass sensor to measure the density of single living cells. By weighing each cell in two fluids of different densities, our technique measures the single-cell mass, volume, and density of approximately 500 cells per hour with a density precision of 0.001 g mL(-1). We observe that the intrinsic cell-to-cell variation in density is nearly 100-fold smaller than the mass or volume variation. As a result, we can measure changes in cell density indicative of cellular processes that would be otherwise undetectable by mass or volume measurements. Here, we demonstrate this with four examples: identifying Plasmodium falciparum malaria-infected erythrocytes in a culture, distinguishing transfused blood cells from a patient's own blood, identifying irreversibly sickled cells in a sickle cell patient, and identifying leukemia cells in the early stages of responding to a drug treatment. These demonstrations suggest that the ability to measure single-cell density will provide valuable insights into cell state for a wide range of biological processes.
Conflict of interest statement
Conflict of interest statement: S.R.M. is a cofounder of Affinity Biosensors and declares competing financial interests.
Figures




References
-
- Maric D, Maric I, Barker JL. Buoyant density gradient fractionation and flow cytometric analysis of embryonic rat cortical neurons and progenitor cells. Methods. 1998;16:247–259. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

