Phosphorylation of GSK-3β mediates intralipid-induced cardioprotection against ischemia/reperfusion injury
- PMID: 21691195
- PMCID: PMC3322241
- DOI: 10.1097/ALN.0b013e318223b8b9
Phosphorylation of GSK-3β mediates intralipid-induced cardioprotection against ischemia/reperfusion injury
Abstract
Background: Intralipid (Sigma, St. Louis, MO), a brand name for the first safe fat emulsion for human use, has been shown to be cardioprotective. However, the mechanism of this protection is not known. The authors investigated the molecular mechanism(s) of Intralipid-induced cardioprotection against ischemia/reperfusion injury, particularly the role of glycogen synthase kinase-3β (GSK-3β) and mitochondrial permeability transition pore in this protective action.
Methods: In vivo rat hearts or isolated Langendorff-perfused mouse hearts were subjected to ischemia followed by reperfusion with Intralipid (1% in ex vivo and one bolus of 20% in in vivo) or vehicle. The hemodynamic function, infarct size, threshold for the opening of mitochondrial permeability transition pore, and phosphorylation levels of protein kinase B (Akt)/extracellular signal regulating kinase (ERK)/GSK-3β were measured.
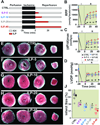
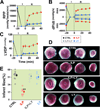
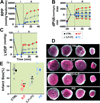
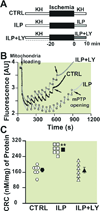
Results: Administration of Intralipid at the onset of reperfusion resulted in approximately 70% reduction in infarct size in the in vivo rat model. Intralipid also significantly improved functional recovery of isolated Langendorff-perfused mouse hearts as the rate pressure product was increased from 2,999 ± 863 mmHg*beats/min in the control group to 13,676 ± 611 mmHg*beats/min (mean±SEM) and the infarct size was markedly smaller (18.3 ± 2.4% vs. 54.8 ± 2.9% in the control group, P < 0.01). The Intralipid-induced cardioprotection was fully abolished by LY294002, a specific inhibitor of PI3K, but only partially by PD98059, a specific ERK inhibitor. Intralipid also increased the phosphorylation levels of Akt/ERK1/glycogen synthase kinase-3β by eightfold, threefold, and ninefold, respectively. The opening of mitochondrial permeability transition pore was inhibited by Intralipid because calcium retention capacity was higher in the Intralipid group (274.3 ± 8.4 nM/mg vs. 168.6 ± 9.6 nM/mg in the control group).
Conclusions: Postischemic treatment with Intralipid inhibits the opening of mitochondiral permeability transition pore and protects the heart through glycogen synthase kinase-3β via PI3K/Akt/ERK pathways.
Conflict of interest statement
Figures



Comment in
-
From local to global: lipid emulsion (intralipid) makes a move.Anesthesiology. 2011 Aug;115(2):226-8. doi: 10.1097/ALN.0b013e318223b8f4. Anesthesiology. 2011. PMID: 21654493 No abstract available.
-
Intralipid: the new magic bullet in cardioprotection?Anesthesiology. 2013 May;118(5):1237-8. doi: 10.1097/ALN.0b013e31828bac35. Anesthesiology. 2013. PMID: 23612140 Free PMC article. No abstract available.
-
In reply.Anesthesiology. 2013 May;118(5):1238-40. doi: 10.1097/ALN.0b013e31828baec5. Anesthesiology. 2013. PMID: 23612141 No abstract available.
-
Current evidence supports use of lipid rescue therapy in local anaesthetic systemic toxicity.Acta Anaesthesiol Scand. 2017 Apr;61(4):365-368. doi: 10.1111/aas.12870. Acta Anaesthesiol Scand. 2017. PMID: 28251603 No abstract available.
References
-
- Christian TF, Schwartz RS, Gibbons RJ. Determinants of infarct size in reperfusion therapy for acute myocardial infarction. Circulation. 1992;86:81–90. - PubMed
-
- Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: Comparison with ischemic preconditioning. Am.J.Physiol Heart Circ.Physiol. 2003;285:H579–H588. - PubMed
-
- Bopassa JC, Ferrera R, Gateau-Roesch O, Couture-Lepetit E, Ovize M. PI 3-kinase regulates the mitochondrial transition pore in controlled reperfusion and postconditioning. Cardiovas Res. 2006;69:178–185. - PubMed
-
- Cohen MV, Yang XM, Downey JM. The pH hypothesis of postconditioning: Staccato reperfusion reintroduces oxygen and perpetuates myocardial acidosis. Circulation. 2007;115:1895–1903. - PubMed
-
- Tamareille S, Ghaboura N, Treguer F, Khachman D, Croue A, Henrion D, Furber A, Prunier F. Myocardial reperfusion injury management: Erythropoietin compared with postconditioning. Am.J.Physiol Heart Circ.Physiol. 2009;297:H2035–H2043. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

