Mechanisms of disease: pulmonary arterial hypertension
- PMID: 21691314
- PMCID: PMC7097518
- DOI: 10.1038/nrcardio.2011.87
Mechanisms of disease: pulmonary arterial hypertension
Abstract
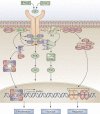
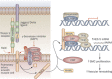
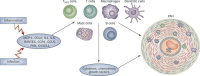
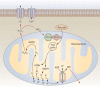
Our understanding of, and approach to, pulmonary arterial hypertension has undergone a paradigm shift in the past decade. Once a condition thought to be dominated by increased vasoconstrictor tone and thrombosis, pulmonary arterial hypertension is now seen as a vasculopathy in which structural changes driven by excessive vascular cell growth and inflammation, with recruitment and infiltration of circulating cells, play a major role. Perturbations of a number of molecular mechanisms have been described, including pathways involving growth factors, cytokines, metabolic signaling, elastases, and proteases, that may underlie the pathogenesis of the disease. Elucidating their contribution to the pathophysiology of pulmonary arterial hypertension could offer new drug targets. The role of progenitor cells in vascular repair is also under active investigation. The right ventricular response to increased pressure load is recognized as critical to survival and the molecular mechanisms involved are attracting increasing interest. The challenge now is to integrate this new knowledge and explore how it can be used to categorize patients by molecular phenotype and tailor treatment more effectively.
Conflict of interest statement
R. T. Schermuly declares he has been a consultant for Actelion, Bayer Healthcare, Ergonex, and Solvay Pharmaceuticals, and has received speakers bureau (honoraria) from Actelion, Bayer Healthcare, GlaxoSmithKlein, Lilly, Solvay Pharmaceuticals, and Pfizer. R. T. Schermuly also declares he has received grant/research support from Actelion, Bayer Healthcare, Ergonex, Excellence Cluster Cardiopulmonary System, German Research Foundation, Gilead, Novartis, Solvay Pharmaceuticals, Pfizer, and the University of Giessen and Marburg Lung Center.
H. A. Ghofrani declares he has been a consultant for Bayer Healthcare, Ergonex, GlaxoSmithKlein, Novartis, and Pfizer, and has received speakers bureau (honoraria) from Bayer Healthcare, GlaxoSmithKlein, Novartis, and Pfizer. H. A. Ghofrani also declares he has received grant/research support from Bayer Healthcare, Excellence Cluster Cardiopulmonary System, German Research Foundation, Novartis, Pfizer, and the University of Giessen and Marburg Lung Center.
M. R. Wilkins declares he has been a consultant for Biomarin and Pfizer, has received speakers bureau (honoraria) from Bayer Healthcare, GlaxoSmithKlein, and Pfizer, and has received grant/research support from Biomarin.
F. Grimminger declares he has been a consultant for Bayer Healthcare, has received speakers bureau (honoraria) from Actelion, Encysive, and Pfizer, and has received grant/research support from Actelion, Excellence Cluster Cardiopulmonary System, GlaxoSmithKlein, German Research Foundation, Novartis, Parexel International GmbH, Pfizer, and the University of Giessen and Marburg Lung Center.
Figures

References
-
- Simonneau G. Updated clinical classification of pulmonary hypertension. J. Am. Coll. Cardiol. 2009;54:S43–S54. - PubMed
-
- dos Santos Fernandes CJ. Survival in schistosomiasis-associated pulmonary arterial hypertension. J. Am. Coll. Cardiol. 2010;56:715–720. - PubMed
-
- Lapa M. Cardiopulmonary manifestations of hepatosplenic schistosomiasis. Circulation. 2009;119:1518–1523. - PubMed
-
- Butrous G, Ghofrani HA, Grimminger F. Pulmonary vascular disease in the developing world. Circulation. 2008;118:1758–1766. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

