Glucose, not glutamine, is the dominant energy source required for proliferation and survival of head and neck squamous carcinoma cells
- PMID: 21692052
- PMCID: PMC3135768
- DOI: 10.1002/cncr.25868
Glucose, not glutamine, is the dominant energy source required for proliferation and survival of head and neck squamous carcinoma cells
Abstract
Background: Tumor metabolism is an essential contributor to disease progression and response to treatment. An understanding of the metabolic phenotype of head and neck squamous cell carcinoma (HNSCC) will allow the development of appropriate antimetabolic strategies for this tumor type.
Methods: A panel of 15 HNSCC cell lines was assayed for glucose and glutamine dependence and sensitivity to metabolic inhibitors. In addition, broad-spectrum metabolomic analysis using mass spectrometry/liquid chromatography was combined with individual measurements of reducing potential, adenosine triphosphate, and lactate production to characterize cellular metabolic phenotypes.
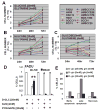
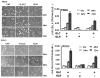
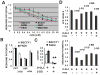
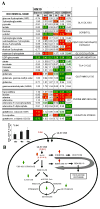
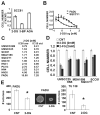
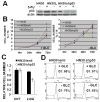
Results: HNSCC energy and reducing potential levels closely mirrored extracellular glucose concentrations. Glucose starvation induced cell death despite the activation of secondary energetic pathways. Conversely, glutamine was not required for HNSCC survival and did not serve as a significant source of energy. 2-deoxyglucose (2-DG) and its fluorinated derivative decreased glycolytic and Krebs cycle activity, cellular energy, and reducing potential and inhibited HNSCC cell proliferation. 2-DG effects were potentiated by the addition of metformin, but not by inhibitors of the pentose phosphate pathway or glutaminolysis. Despite dependence on glucose catabolism, the authors identified a subset of cell lines with relative resistance to starvation. Exploration of 1 such cell line (HN30) suggested that the presence of wild-type p53 can partially protect tumor cells from glucose starvation.
Conclusions: HNSCC tumor cells are dependent on glucose, not glutamine, for energy production and survival, providing a rationale for treatment strategies that target glucose catabolism. However, antimetabolic strategies may need to be tailored to the tumor background, more specifically, p53 status.
Copyright © 2011 American Cancer Society.
Figures


References
-
- Pignon JP, le Maitre A, Maillard E, Bourhis J. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92(1):4–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

