A survey of oxidative paracatalytic reactions catalyzed by enzymes that generate carbanionic intermediates: implications for ROS production, cancer etiology, and neurodegenerative diseases
- PMID: 21692372
- PMCID: PMC3563061
- DOI: 10.1002/9780470920541.ch7
A survey of oxidative paracatalytic reactions catalyzed by enzymes that generate carbanionic intermediates: implications for ROS production, cancer etiology, and neurodegenerative diseases
Figures
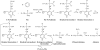

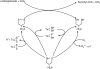
Similar articles
-
Enzyme-catalyzed side reactions with molecular oxygen may contribute to cell signaling and neurodegenerative diseases.Neurochem Res. 2007 Apr-May;32(4-5):871-91. doi: 10.1007/s11064-006-9239-z. Epub 2007 Mar 7. Neurochem Res. 2007. PMID: 17342415 Review.
-
Oxygen reactivity with pyridoxal 5'-phosphate enzymes: biochemical implications and functional relevance.Amino Acids. 2020 Aug;52(8):1089-1105. doi: 10.1007/s00726-020-02885-6. Epub 2020 Aug 25. Amino Acids. 2020. PMID: 32844248 Free PMC article. Review.
-
Oxidative Stress: A Key Modulator in Neurodegenerative Diseases.Molecules. 2019 Apr 22;24(8):1583. doi: 10.3390/molecules24081583. Molecules. 2019. PMID: 31013638 Free PMC article. Review.
-
Proton transfer at carbon.Curr Opin Chem Biol. 2001 Dec;5(6):626-33. doi: 10.1016/s1367-5931(01)00258-7. Curr Opin Chem Biol. 2001. PMID: 11738171 Review.
-
Mechanism of dioxygen activation in 2-oxoglutarate-dependent enzymes: a hybrid DFT study.Chemistry. 2004 Feb 20;10(4):1031-41. doi: 10.1002/chem.200305306. Chemistry. 2004. PMID: 14978830
Cited by
-
Disruption of an Active Site Network Leads to Activation of C2α-Lactylthiamin Diphosphate on the Antibacterial Target 1-Deoxy-d-xylulose-5-phosphate Synthase.Biochemistry. 2024 Mar 5;63(5):671-687. doi: 10.1021/acs.biochem.3c00735. Epub 2024 Feb 23. Biochemistry. 2024. PMID: 38393327 Free PMC article.
-
The 2-oxoacid dehydrogenase complexes in mitochondria can produce superoxide/hydrogen peroxide at much higher rates than complex I.J Biol Chem. 2014 Mar 21;289(12):8312-25. doi: 10.1074/jbc.M113.545301. Epub 2014 Feb 10. J Biol Chem. 2014. PMID: 24515115 Free PMC article.
-
The number of catalytic cycles in an enzyme's lifetime and why it matters to metabolic engineering.Proc Natl Acad Sci U S A. 2021 Mar 30;118(13):e2023348118. doi: 10.1073/pnas.2023348118. Proc Natl Acad Sci U S A. 2021. PMID: 33753504 Free PMC article.
-
Enzyme-catalyzed side reactions with molecular oxygen may contribute to cell signaling and neurodegenerative diseases.Neurochem Res. 2007 Apr-May;32(4-5):871-91. doi: 10.1007/s11064-006-9239-z. Epub 2007 Mar 7. Neurochem Res. 2007. PMID: 17342415 Review.
-
Rethinking the PDH Bypass and GABA Shunt as Thiamin-Deficiency Workarounds.Plant Physiol. 2019 Oct;181(2):389-393. doi: 10.1104/pp.19.00857. Epub 2019 Aug 13. Plant Physiol. 2019. PMID: 31409697 Free PMC article.
References
-
- Healy MJ, Christen P. Reaction of the carbanionic aldolase-substrate intermediate with tetranitromethane: identification of the products, hydroxypyruvaldehyde phosphate and D-5-ketofructose 1,6-diphosphate. J. Am. Chem. Soc. 1972;94:7911–7916. - PubMed
-
- Healy MJ, Christen P. Mechanistic probes for enzymatic reactions: oxidation–reduction indicators as oxidants of intermediary carbanions (studies with aldolase, aspartate aminotransferase, pyruvate decarboxylase, and 6-phosphogluconate dehydrogenase) Biochemistry. 1973;12:35–41. - PubMed
-
- Christen P, Anderson TK, Healy MJ. H2O2 oxidizes an aldolase dihydroxyacetone phosphate intermediate to hydroxymethylglyoxal phosphate. Experientia. 1974;30:603–605. - PubMed
-
- Christen P, Gasser A. Oxidation of the carbanion intermediate of transaldolase by hexacyanoferrate(III) J. Biol. Chem. 1976;251:4220–4223. - PubMed
-
- Christen P, Cogoli-Greuter M, Healy MJ, Lubini D. Specific irreversible inhibition of enzymes concomitant to the oxidation of carbanionic enzyme– substrate intermediates by hexacyanoferrate(III) Eur. J. Biochem. 1976;63:223–231. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
