Hyperpolarized [1-13C]-ascorbic and dehydroascorbic acid: vitamin C as a probe for imaging redox status in vivo
- PMID: 21692446
- PMCID: PMC3144679
- DOI: 10.1021/ja2045925
Hyperpolarized [1-13C]-ascorbic and dehydroascorbic acid: vitamin C as a probe for imaging redox status in vivo
Abstract
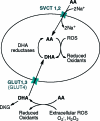
Dynamic nuclear polarization (DNP) of (13)C-labeled metabolic substrates in vitro and their subsequent intravenous administration allow both the location of the hyperpolarized substrate and the dynamics of its subsequent conversion into other metabolic products to be detected in vivo. We report here the hyperpolarization of [1-(13)C]-ascorbic acid (AA) and [1-(13)C]-dehydroascorbic acid (DHA), the reduced and oxidized forms of vitamin C, respectively, and evaluate their performance as probes of tumor redox state. Solution-state polarization of 10.5 ± 1.3% was achieved for both forms at pH 3.2, whereas at pH 7.0, [1-(13)C]-AA retained polarization of 5.1 ± 0.6% and [1-(13)C]-DHA retained 8.2 ± 1.1%. The spin-lattice relaxation times (T(1)'s) for these labeled nuclei are long at 9.4 T: 15.9 ± 0.7 s for AA and 20.5 ± 0.9 s for DHA. Extracellular oxidation of [1-(13)C]-AA and intracellular reduction of [1-(13)C]-DHA were observed in suspensions of murine lymphoma cells. The spontaneous reaction of DHA with the cellular antioxidant glutathione was monitored in vitro and was approximately 100-fold lower than the rate observed in cell suspensions, indicating enzymatic involvement in the intracellular reduction. [1-(13)C]-DHA reduction was also detected in lymphoma tumors in vivo. In contrast, no detectable oxidation of [1-(13)C]-AA was measured in the same tumors, consistent with the notion that tumors maintain a reduced microenvironment. This study demonstrates that hyperpolarized (13)C-labeled vitamin C could be used as a noninvasive biomarker of redox status in vivo, which has the potential to translate to the clinic.
Figures



References
-
- Day S. E.; Kettunen M. I.; Gallagher F. A.; Hu D. E.; Lerche M.; Wolber J.; Golman K.; Ardenkjaer-Larsen J. H.; Brindle K. M. Nat. Med. 2007, 13, 1382–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

