Sequential activation and deactivation of protein function using spectrally differentiated caged phosphoamino acids
- PMID: 21692531
- PMCID: PMC3146339
- DOI: 10.1021/ja2028074
Sequential activation and deactivation of protein function using spectrally differentiated caged phosphoamino acids
Abstract
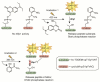
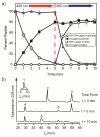
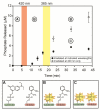
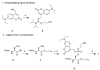
Photolabile caging groups, including the 1-(2-nitrophenyl)ethyl (NPE) group, have been applied to probe many biological processes, including protein phosphorylation. Although studies with NPE-caged phosphoamino acids have provided valuable information, these investigations have been limited to the use of only one caged species in a single experiment. To expand the scope of these tools, we have developed an approach for sequentially uncaging two different phosphopeptides in one system, enabling interrogation of multiple phosphorylation events. We present the synthesis of [7-(diethylamino)coumarin-4-yl]methyl (DEACM)-caged phosphorylated serine, threonine, and tyrosine building blocks for Fmoc-based solid-phase peptide synthesis to allow convenient incorporation of these residues into peptides and proteins. Exposure of DEACM- and NPE-caged phosphopeptides to 420 nm light selectively releases the DEACM group without affecting the NPE-caged peptide. This then enables a subsequent irradiation event at 365 nm to remove the NPE group and liberate a second phosphopeptide. We demonstrate the versatility of this general sequential uncaging approach by applying it to control Wip1 phosphatase with two wavelengths of light.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

