Blood pressure long term regulation: a neural network model of the set point development
- PMID: 21693057
- PMCID: PMC3160418
- DOI: 10.1186/1475-925X-10-54
Blood pressure long term regulation: a neural network model of the set point development
Abstract
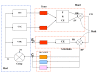
Background: The notion of the nucleus tractus solitarius (NTS) as a comparator evaluating the error signal between its rostral neural structures (RNS) and the cardiovascular receptor afferents into it has been recently presented. From this perspective, stress can cause hypertension via set point changes, so offering an answer to an old question. Even though the local blood flow to tissues is influenced by circulating vasoactive hormones and also by local factors, there is yet significant sympathetic control. It is well established that the state of maturation of sympathetic innervation of blood vessels at birth varies across animal species and it takes place mostly during the postnatal period. During ontogeny, chemoreceptors are functional; they discharge when the partial pressures of oxygen and carbon dioxide in the arterial blood are not normal.
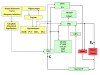
Methods: The model is a simple biological plausible adaptative neural network to simulate the development of the sympathetic nervous control. It is hypothesized that during ontogeny, from the RNS afferents to the NTS, the optimal level of each sympathetic efferent discharge is learned through the chemoreceptors' feedback. Its mean discharge leads to normal oxygen and carbon dioxide levels in each tissue. Thus, the sympathetic efferent discharge sets at the optimal level if, despite maximal drift, the local blood flow is compensated for by autoregulation. Such optimal level produces minimum chemoreceptor output, which must be maintained by the nervous system. Since blood flow is controlled by arterial blood pressure, the long-term mean level is stabilized to regulate oxygen and carbon dioxide levels. After development, the cardiopulmonary reflexes play an important role in controlling efferent sympathetic nerve activity to the kidneys and modulating sodium and water excretion.
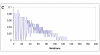
Results: Starting from fixed RNS afferents to the NTS and random synaptic weight values, the sympathetic efferents converged to the optimal values. When learning was completed, the output from the chemoreceptors became zero because the sympathetic efferents led to normal partial pressures of oxygen and carbon dioxide.
Conclusions: We introduce here a simple simulating computational theory to study, from a neurophysiologic point of view, the sympathetic development of cardiovascular regulation due to feedback signals sent off by cardiovascular receptors. The model simulates, too, how the NTS, as emergent property, acts as a comparator and how its rostral afferents behave as set point.
Figures






References
-
- Zanutto BS, Valentinuzzi ME, Segura ET. Neural set point for the control of arterial pressure: role of the nucleus tractus solitarius (review) BioMedical Engineering OnLine. 2010;9:4. doi: 10.1186/1475-925X-9-4. http://www.biomedical-engineering-online.com/content/9/1/4 - DOI - PMC - PubMed
-
- Valentinuzzi ME, Powell T, Hoff HE, Geddes LA, Posey JA. Control parameters of the blood pressure regulatory system (Part II): Open-loop gain, reference pressure and basal heart rate. Med & Biol Eng. 1972;10:596–608. - PubMed
-
- Shepherd JT. In: Handbook of Physiology: The Cardiovascular System. Shepherd JT, Abboud FM, Geiber SR, editor. Baltimore: Waverly Press; 1983. Circulation to skeletal muscle; pp. 319–370.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

