Indole-3-carbinol downregulation of telomerase gene expression requires the inhibition of estrogen receptor-alpha and Sp1 transcription factor interactions within the hTERT promoter and mediates the G1 cell cycle arrest of human breast cancer cells
- PMID: 21693539
- PMCID: PMC3165124
- DOI: 10.1093/carcin/bgr116
Indole-3-carbinol downregulation of telomerase gene expression requires the inhibition of estrogen receptor-alpha and Sp1 transcription factor interactions within the hTERT promoter and mediates the G1 cell cycle arrest of human breast cancer cells
Abstract
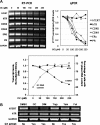
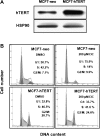
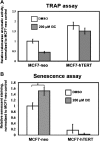
Indole-3-carbinol (I3C), a naturally occurring hydrolysis product of glucobrassicin from cruciferous vegetables such as broccoli, cabbage and Brussels sprouts, is an anticancer phytochemical that triggers complementary sets of antiproliferative pathways to induce a cell cycle arrest of estrogen-responsive MCF7 breast cancer cells. I3C strongly downregulated transcript expression of the catalytic subunit of the human telomerase (hTERT) gene, which correlated with the dose-dependent indole-mediated G(1) cell cycle arrest without altering the transcript levels of the RNA template (hTR) for telomerase elongation. Exogenous expression of hTERT driven by a constitutive promoter prevented the I3C-induced cell cycle arrest and rescued the I3C inhibition of telomerase enzymatic activity and activation of cellular senescence. Time course studies showed that I3C downregulated expression of estrogen receptor-alpha (ERα) and cyclin-dependent kinase-6 transcripts levels (which is regulated through the Sp1 transcription factor) prior to the downregulation of hTERT suggesting a mechanistic link. Chromatin immunoprecipitation assays demonstrated that I3C disrupted endogenous interactions of both ERα and Sp1 with an estrogen response element-Sp1 composite element within the hTERT promoter. I3C inhibited 17β-estradiol stimulated hTERT expression and stimulated the production of threonine-phosphorylated Sp1, which inhibits Sp1-DNA interactions. Exogenous expression of both ERα and Sp1, but not either alone, in MCF7 cells blocked the I3C-mediated downregulation of hTERT expression. These results demonstrate that I3C disrupts the combined ERα- and Sp1-driven transcription of hTERT gene expression, which plays a significant role in the I3C-induced cell cycle arrest of human breast cancer cells.
Figures


Similar articles
-
1-Benzyl-indole-3-carbinol is a novel indole-3-carbinol derivative with significantly enhanced potency of anti-proliferative and anti-estrogenic properties in human breast cancer cells.Chem Biol Interact. 2010 Aug 5;186(3):255-66. doi: 10.1016/j.cbi.2010.05.015. Epub 2010 Jun 2. Chem Biol Interact. 2010. PMID: 20570586 Free PMC article.
-
Indole-3-carbinol selectively uncouples expression and activity of estrogen receptor subtypes in human breast cancer cells.Mol Endocrinol. 2006 Dec;20(12):3070-82. doi: 10.1210/me.2005-0263. Epub 2006 Aug 10. Mol Endocrinol. 2006. PMID: 16901971
-
Indole-3-carbinol disrupts estrogen receptor-alpha dependent expression of insulin-like growth factor-1 receptor and insulin receptor substrate-1 and proliferation of human breast cancer cells.Mol Cell Endocrinol. 2012 Nov 5;363(1-2):74-84. doi: 10.1016/j.mce.2012.07.008. Epub 2012 Jul 24. Mol Cell Endocrinol. 2012. PMID: 22835548 Free PMC article.
-
Indole-3-carbinol and 3-3'-diindolylmethane antiproliferative signaling pathways control cell-cycle gene transcription in human breast cancer cells by regulating promoter-Sp1 transcription factor interactions.J Nutr. 2003 Jul;133(7 Suppl):2448S-2455S. doi: 10.1093/jn/133.7.2448S. J Nutr. 2003. PMID: 12840223 Review.
-
Molecular targets and anticancer potential of indole-3-carbinol and its derivatives.Cell Cycle. 2005 Sep;4(9):1201-15. doi: 10.4161/cc.4.9.1993. Epub 2005 Sep 6. Cell Cycle. 2005. PMID: 16082211 Review.
Cited by
-
Inhibition of telomerase activity by oleanane triterpenoid CDDO-Me in pancreatic cancer cells is ROS-dependent.Molecules. 2013 Mar 13;18(3):3250-65. doi: 10.3390/molecules18033250. Molecules. 2013. PMID: 23486104 Free PMC article.
-
Indole-3-carbinol inhibits nasopharyngeal carcinoma growth through cell cycle arrest in vivo and in vitro.PLoS One. 2013 Dec 16;8(12):e82288. doi: 10.1371/journal.pone.0082288. eCollection 2013. PLoS One. 2013. Retraction in: PLoS One. 2022 Oct 4;17(10):e0275881. doi: 10.1371/journal.pone.0275881. PMID: 24358165 Free PMC article. Retracted.
-
Telomerase Inhibitors from Natural Products and Their Anticancer Potential.Int J Mol Sci. 2017 Dec 21;19(1):13. doi: 10.3390/ijms19010013. Int J Mol Sci. 2017. PMID: 29267203 Free PMC article. Review.
-
Telomerase reverse transcriptase (TERT) is a therapeutic target of oleanane triterpenoid CDDO-Me in prostate cancer.Molecules. 2012 Dec 11;17(12):14795-809. doi: 10.3390/molecules171214795. Molecules. 2012. PMID: 23519253 Free PMC article.
-
Antiproliferative Effects of Ferulic, Coumaric, and Caffeic Acids in HepG2 Cells by hTERT Downregulation.Adv Pharmacol Pharm Sci. 2022 Oct 28;2022:1850732. doi: 10.1155/2022/1850732. eCollection 2022. Adv Pharmacol Pharm Sci. 2022. PMID: 36341080 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

