Overlapping signals for translational regulation and packaging of influenza A virus segment 2
- PMID: 21693560
- PMCID: PMC3177217
- DOI: 10.1093/nar/gkr487
Overlapping signals for translational regulation and packaging of influenza A virus segment 2
Abstract
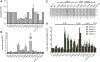
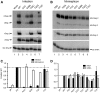
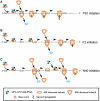
Influenza A virus segment 2 mRNA expresses three polypeptides: PB1, PB1-F2 and PB1-N40, from AUGs 1, 4 and 5 respectively. Two short open reading frames (sORFs) initiated by AUGs 2 and 3 are also present. To understand translational regulation in this system, we systematically mutated AUGs 1-4 and monitored polypeptide synthesis from plasmids and recombinant viruses. This identified sORF2 as a key regulatory element with opposing effects on PB1-F2 and PB1-N40 expression. We propose a model in which AUGs 1-4 are accessed by leaky ribosomal scanning, with sORF2 repressing synthesis of downstream PB1-F2. However, sORF2 also up-regulates PB1-N40 expression, most likely by a reinitiation mechanism that permits skipping of AUG4. Surprisingly, we also found that in contrast to plasmid-driven expression, viruses with improved AUG1 initiation contexts produced less PB1 in infected cells and replicated poorly, producing virions with elevated particle:PFU ratios. Analysis of the genome content of virus particles showed reduced packaging of the mutant segment 2 vRNAs. Overall, we conclude that segment 2 mRNA translation is regulated by a combination of leaky ribosomal scanning and reinitiation, and that the sequences surrounding the PB1 AUG codon are multifunctional, containing overlapping signals for translation initiation and for segment-specific packaging.
Figures

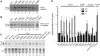




Similar articles
-
The Influenza A PB1-F2 and N40 Start Codons Are Contained within an RNA Pseudoknot.Biochemistry. 2015 Jun 9;54(22):3413-5. doi: 10.1021/bi501564d. Epub 2015 May 27. Biochemistry. 2015. PMID: 25996464 Free PMC article.
-
A complicated message: Identification of a novel PB1-related protein translated from influenza A virus segment 2 mRNA.J Virol. 2009 Aug;83(16):8021-31. doi: 10.1128/JVI.00826-09. Epub 2009 Jun 3. J Virol. 2009. PMID: 19494001 Free PMC article.
-
Behaviour of influenza A viruses differentially expressing segment 2 gene products in vitro and in vivo.J Gen Virol. 2012 Apr;93(Pt 4):840-849. doi: 10.1099/vir.0.039966-0. Epub 2011 Dec 21. J Gen Virol. 2012. PMID: 22190016
-
Translational termination-reinitiation in RNA viruses.Biochem Soc Trans. 2010 Dec;38(6):1558-64. doi: 10.1042/BST0381558. Biochem Soc Trans. 2010. PMID: 21118126 Review.
-
Current knowledge on PB1-F2 of influenza A viruses.Med Microbiol Immunol. 2011 May;200(2):69-75. doi: 10.1007/s00430-010-0176-8. Epub 2010 Oct 16. Med Microbiol Immunol. 2011. PMID: 20953627 Review.
Cited by
-
The Influenza A PB1-F2 and N40 Start Codons Are Contained within an RNA Pseudoknot.Biochemistry. 2015 Jun 9;54(22):3413-5. doi: 10.1021/bi501564d. Epub 2015 May 27. Biochemistry. 2015. PMID: 25996464 Free PMC article.
-
Proteomic Analysis of Differentially Expressed Proteins in A549 Cells Infected with H9N2 Avian Influenza Virus.Int J Mol Sci. 2025 Jan 14;26(2):657. doi: 10.3390/ijms26020657. Int J Mol Sci. 2025. PMID: 39859371 Free PMC article.
-
MicroRNAs affect GPCR and Ion channel genes needed for influenza replication.J Gen Virol. 2021 Nov;102(11):001691. doi: 10.1099/jgv.0.001691. J Gen Virol. 2021. PMID: 34787540 Free PMC article.
-
Influenza virus PB1 and neuraminidase gene segments can cosegregate during vaccine reassortment driven by interactions in the PB1 coding region.J Virol. 2014 Aug;88(16):8971-80. doi: 10.1128/JVI.01022-14. Epub 2014 May 28. J Virol. 2014. PMID: 24872588 Free PMC article.
-
Influenza A Virus M2 Protein: Roles from Ingress to Egress.Int J Mol Sci. 2017 Dec 7;18(12):2649. doi: 10.3390/ijms18122649. Int J Mol Sci. 2017. PMID: 29215568 Free PMC article. Review.
References
-
- Portela A, Digard P. The influenza virus nucleoprotein: a multifunctional RNA-binding protein pivotal to virus replication. J. Gen. Virol. 2002;83:723–734. - PubMed
-
- Neumann G, Brownlee GG, Fodor E, Kawaoka Y. Orthomyxovirus replication, transcription, and polyadenylation. Curr. Top. Microbiol. Immunol. 2004;283:121–143. - PubMed
-
- Hutchinson EC, von Kirchbach JC, Gog JR, Digard P. Genome packaging in influenza A virus. J. Gen. Virol. 2010;91:313–328. - PubMed
-
- Naffakh N, Tomoiu A, Rameix-Welti MA, van der Werf S. Host restriction of avian influenza viruses at the level of the ribonucleoproteins. Annu. Rev. Microbiol. 2008;62:403–424. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

