TGFB1 disrupts the angiogenic potential of microvascular endothelial cells of the corpus luteum
- PMID: 21693577
- PMCID: PMC6518331
- DOI: 10.1242/jcs.084558
TGFB1 disrupts the angiogenic potential of microvascular endothelial cells of the corpus luteum
Abstract
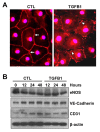
Cyclical formation and regression of the ovarian corpus luteum is required for reproduction. During luteal regression, the microvasculature of the corpus luteum is extensively disrupted. Prostaglandin F2α, a primary signal for luteal regression, induces the expression of transforming growth factor β1 (TGFB1) in the corpus luteum. This study determined the actions of TGFB1 on microvascular endothelial cells isolated from the bovine corpus luteum (CLENDO cells). We hypothesized that TGFB1 participates in the disruption of the microvasculature during luteal regression. TGFB1 activated the canonical SMAD signaling pathway in CLENDO cells. TGFB1 (1 ng/ml) significantly reduced both basal and fetal-calf-serum-stimulated DNA synthesis, without reducing cell viability. TGFB1 also significantly reduced CLENDO cell transwell migration and disrupted the formation of capillary-like structures when CLENDO cells were plated on Matrigel. By contrast, CLENDO cells plated on fibrillar collagen I gels did not form capillary-like structures and TGFB1 induced cell death. Additionally, TGFB1 caused loss of VE-cadherin from cellular junctions and loss of cell-cell contacts, and increased the permeability of confluent CLENDO cell monolayers. These studies demonstrate that TGFB1 acts directly on CLENDO cells to limit endothelial cell function and suggest that TGFB1 might act in the disassembly of capillaries observed during luteal regression.
Figures







References
-
- Alvarez D. F., Huang L., King J. A., ElZarrad M. K., Yoder M. C., Stevens T. (2008). Lung microvascular endothelium is enriched with progenitor cells that exhibit vasculogenic capacity. Am J. Physiol. Lung Cell. Mol. Physiol. 294, L419-L430. - PubMed
-
- Behzadian M. A., Wang X. L., Windsor L. J., Ghaly N., Caldwell R. B. (2001). TGF-β increases retinal endothelial cell permeability by increasing MMP-9: possible role of glial cells in endothelial barrier function. Invest. Ophthalmol. Vis. Sci. 42, 85385-85389. - PubMed
-
- Bein K., Odell-Fiddler E. T., Drinane M. (2004). Role of TGF-beta1 and JNK signaling in capillary tube patterning. Am. J. Physiol. Cell Physiol. 287, C1012-C1022. - PubMed
-
- Berisha B., Meyer H. H. D., Schams D. (2010). Effect of prostaglandin F2 Alpha on local luteotropic and angiogenic factors during induced functional luteolysis in the bovine corpus luteum. Biol. Reprod. 82, 940-947. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

