R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/beta-catenin signaling
- PMID: 21693646
- PMCID: PMC3136304
- DOI: 10.1073/pnas.1106083108
R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/beta-catenin signaling
Abstract
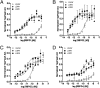
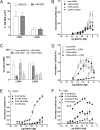
The Wnt/β-catenin signaling system plays essential roles in embryonic development and in the self-renewal and maintenance of adult stem cells. R-spondins (RSPOs) are a group of secreted proteins that enhance Wnt/β-catenin signaling and have pleiotropic functions in development and stem cell growth. LGR5, an orphan receptor of the G protein-coupled receptor (GPCR) superfamily, is specifically expressed in stem cells of the intestinal crypt and hair follicle. Knockout of LGR5 in the mouse results in neonatal lethality. LGR4, a receptor closely related to LGR5, also has essential roles in development, as its knockout leads to reduced viability and retarded growth. Overexpression of both receptors has been reported in several types of cancer. Here we demonstrate that LGR4 and LGR5 bind the R-spondins with high affinity and mediate the potentiation of Wnt/β-catenin signaling by enhancing Wnt-induced LRP6 phosphorylation. Interestingly, neither receptor is coupled to heterotrimeric G proteins or to β-arrestin when stimulated by the R-spondins, indicating a unique mechanism of action. The findings provide a basis for stem cell-specific effects of Wnt/β-catenin signaling and for the broad range of functions LGR4, LGR5, and the R-spondins have in normal and malignant growth.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Barker N, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. - PubMed
-
- Jaks V, et al. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genet. 2008;40:1291–1299. - PubMed
-
- McDonald T, et al. Identification and cloning of an orphan G protein-coupled receptor of the glycoprotein hormone receptor subfamily. Biochem Biophys Res Commun. 1998;247:266–270. - PubMed
-
- Hsu SY, Liang SG, Hsueh AJ. Characterization of two LGR genes homologous to gonadotropin and thyrotropin receptors with extracellular leucine-rich repeats and a G protein-coupled, seven-transmembrane region. Mol Endocrinol. 1998;12:1830–1845. - PubMed
-
- Hermey G, Methner A, Schaller HC, Hermans-Borgmeyer I. Identification of a novel seven-transmembrane receptor with homology to glycoprotein receptors and its expression in the adult and developing mouse. Biochem Biophys Res Commun. 1999;254:273–279. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

