Developmental analysis of a Medicago truncatula smooth leaf margin1 mutant reveals context-dependent effects on compound leaf development
- PMID: 21693694
- PMCID: PMC3160044
- DOI: 10.1105/tpc.111.085464
Developmental analysis of a Medicago truncatula smooth leaf margin1 mutant reveals context-dependent effects on compound leaf development
Abstract
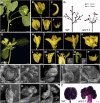
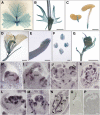
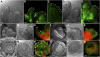
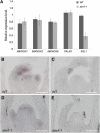
Compound leaf development requires highly regulated cell proliferation, differentiation, and expansion patterns. We identified loss-of-function alleles at the SMOOTH LEAF MARGIN1 (SLM1) locus in Medicago truncatula, a model legume species with trifoliate adult leaves. SLM1 encodes an auxin efflux carrier protein and is the ortholog of Arabidopsis thaliana PIN-FORMED1 (PIN1). Auxin distribution is impaired in the slm1 mutant, resulting in pleiotropic phenotypes in different organs. The most striking change in slm1 is the increase in the number of terminal leaflets and a simultaneous reduction in the number of lateral leaflets, accompanied by reduced expression of SINGLE LEAFLET1 (SGL1), an ortholog of LEAFY. Characterization of the mutant indicates that distinct developmental domains exist in the formation of terminal and lateral leaflets. In contrast with the pinnate compound leaves in the wild type, the slm1 sgl1 double mutant shows nonpeltately palmate leaves, suggesting that the terminal leaflet primordium in M. truncatula has a unique developmental mechanism. Further investigations on the development of leaf serrations reveal different ontogenies between distal serration and marginal serration formation as well as between serration and leaflet formation. These data suggest that regulation of the elaboration of compound leaves and serrations is context dependent and tightly correlated with the auxin/SLM1 module in M. truncatula.
Figures




References
-
- Alexander M.P. (1969). Differential staining of aborted and nonaborted pollen. Stain Technol. 44: 117–122 - PubMed
-
- Balla J., Kalousek P., Reinöhl V., Friml J., Procházka S. (2011). Competitive canalization of PIN-dependent auxin flow from axillary buds controls pea bud outgrowth. Plant J. 65: 571–577 - PubMed
-
- Barkoulas M., Hay A., Kougioumoutzi E., Tsiantis M. (2008). A developmental framework for dissected leaf formation in the Arabidopsis relative Cardamine hirsuta. Nat. Genet. 40: 1136–1141 - PubMed
-
- Benková E., Michniewicz M., Sauer M., Teichmann T., Seifertová D., Jürgens G., Friml J. (2003). Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115: 591–602 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

