Promotion of water-mediated carbon removal by nanostructured barium oxide/nickel interfaces in solid oxide fuel cells
- PMID: 21694705
- PMCID: PMC3157151
- DOI: 10.1038/ncomms1359
Promotion of water-mediated carbon removal by nanostructured barium oxide/nickel interfaces in solid oxide fuel cells
Abstract
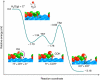
The existing Ni-yttria-stabilized zirconia anodes in solid oxide fuel cells (SOFCs) perform poorly in carbon-containing fuels because of coking and deactivation at desired operating temperatures. Here we report a new anode with nanostructured barium oxide/nickel (BaO/Ni) interfaces for low-cost SOFCs, demonstrating high power density and stability in C(3)H(8), CO and gasified carbon fuels at 750°C. Synchrotron-based X-ray analyses and microscopy reveal that nanosized BaO islands grow on the Ni surface, creating numerous nanostructured BaO/Ni interfaces that readily adsorb water and facilitate water-mediated carbon removal reactions. Density functional theory calculations predict that the dissociated OH from H(2)O on BaO reacts with C on Ni near the BaO/Ni interface to produce CO and H species, which are then electrochemically oxidized at the triple-phase boundaries of the anode. This anode offers potential for ushering in a new generation of SOFCs for efficient, low-emission conversion of readily available fuels to electricity.
Figures

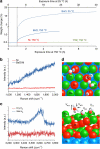
 ] zone axis of the Ni under the BaO island is along the viewing direction. (f) Fourier-filtered [
] zone axis of the Ni under the BaO island is along the viewing direction. (f) Fourier-filtered [ ] zone axis image of the Ni under the BaO island.
] zone axis image of the Ni under the BaO island.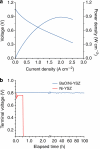



References
-
- Singhal S. C. Advances in solid oxide fuel cell technology. Solid State Ionics 135, 305–313 (2000).
-
- Atkinson A. et al.. Advanced anodes for high-temperature fuel cells. Nat. Mater. 3, 17–27 (2004). - PubMed
-
- la O' G. J. et al.. Catalytic activity enhancement for oxygen reduction on epitaxial perovskite thin films for solid-oxide fuel cells. Angew. Chem. Int. Ed. 49, 5344–5347 (2010). - PubMed
-
- Hibino T. et al.. A low-operating-temperature solid oxide fuel cell in hydrocarbon-air mixtures. Science 288, 2031–2033 (2000). - PubMed
-
- Murray E. P., Tsai T. & Barnett S. A. A direct-methane fuel cell with a ceria-based anode. Nature 400, 649–651 (1999).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

