Cross-species discovery of syncretic drug combinations that potentiate the antifungal fluconazole
- PMID: 21694716
- PMCID: PMC3159983
- DOI: 10.1038/msb.2011.31
Cross-species discovery of syncretic drug combinations that potentiate the antifungal fluconazole
Abstract
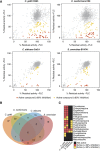
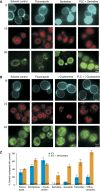
Resistance to widely used fungistatic drugs, particularly to the ergosterol biosynthesis inhibitor fluconazole, threatens millions of immunocompromised patients susceptible to invasive fungal infections. The dense network structure of synthetic lethal genetic interactions in yeast suggests that combinatorial network inhibition may afford increased drug efficacy and specificity. We carried out systematic screens with a bioactive library enriched for off-patent drugs to identify compounds that potentiate fluconazole action in pathogenic Candida and Cryptococcus strains and the model yeast Saccharomyces. Many compounds exhibited species- or genus-specific synergism, and often improved fluconazole from fungistatic to fungicidal activity. Mode of action studies revealed two classes of synergistic compound, which either perturbed membrane permeability or inhibited sphingolipid biosynthesis. Synergistic drug interactions were rationalized by global genetic interaction networks and, notably, higher order drug combinations further potentiated the activity of fluconazole. Synergistic combinations were active against fluconazole-resistant clinical isolates and an in vivo model of Cryptococcus infection. The systematic repurposing of approved drugs against a spectrum of pathogens thus identifies network vulnerabilities that may be exploited to increase the activity and repertoire of antifungal agents.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
-
- Agoston V, Csermely P, Pongor S (2005) Multiple weak hits confuse complex systems: a transcriptional regulatory network as an example. Phys Rev E Stat Nonlin Soft Matter Phys 71: 051909. - PubMed
-
- Arendrup MC, Fisher BT, Zaoutis TE (2009) Invasive fungal infections in the paediatric and neonatal population: diagnostics and management issues. Clin Microbiol Infect 15: 613–624 - PubMed
-
- Baddley JW, Stroud TP, Salzman D, Pappas PG (2001) Invasive mold infections in allogeneic bone marrow transplant recipients. Clin Infect Dis 32: 1319–1324 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

