LyGDI, a novel SHIP-interacting protein, is a negative regulator of FcγR-mediated phagocytosis
- PMID: 21695085
- PMCID: PMC3114867
- DOI: 10.1371/journal.pone.0021175
LyGDI, a novel SHIP-interacting protein, is a negative regulator of FcγR-mediated phagocytosis
Abstract
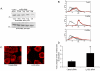
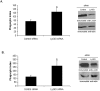
SHIP and SHIP-2 are inositol phosphatases that regulate FcγR-mediated phagocytosis through catalytic as well as non-catalytic mechanisms. In this study we have used two-dimensional fluorescence difference gel electrophoresis (DIGE) analysis to identify downstream signaling proteins that uniquely associate with SHIP or SHIP-2 upon FcγR clustering in human monocytes. We identified LyGDI as a binding partner of SHIP, associating inducibly with the SHIP/Grb2/Shc complex. Immunodepletion and competition experiments with recombinant SHIP domains revealed that Grb2 and the proline-rich domain of SHIP were necessary for SHIP-LyGDI association. Functional studies in primary human monocytes showed that LyGDI sequesters Rac in the cytosol, preventing it from localizing to the membrane. Consistent with this, suppression of LyGDI expression resulted in significantly enhanced FcγR-mediated phagocytosis.
Conflict of interest statement
Figures




References
-
- Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. - PubMed
-
- Pengal RA, Ganesan LP, Fang H, Marsh CB, Anderson CL, et al. SHIP-2 inositol phosphatase is inducibly expressed in human monocytes and serves to regulate Fcgamma receptor-mediated signaling. J Biol Chem. 2003;278:22657–22663. - PubMed
-
- Hejna JA, Saito H, Merkens L Tittle TV, Jakobs PM, et al. Cloning and characterization of a human cDNA (INPPL1) sharing homology with inositol polyphosphate phosphatases. Genomics. 1995;29:285–287. - PubMed
-
- Erneux C, Govaerts C, Communi D, Pesesse X. The diversity and possible functions of the inositol polyphosphate 5-phosphatases. Biochim Biophys Acta. 1998;1436:185–199. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

