Biochemical and functional characterization of the interaction between liprin-α1 and GIT1: implications for the regulation of cell motility
- PMID: 21695141
- PMCID: PMC3113849
- DOI: 10.1371/journal.pone.0020757
Biochemical and functional characterization of the interaction between liprin-α1 and GIT1: implications for the regulation of cell motility
Abstract
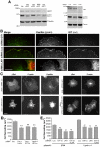
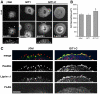
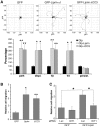
We have previously identified the scaffold protein liprin-α1 as an important regulator of integrin-mediated cell motility and tumor cell invasion. Liprin-α1 may interact with different proteins, and the functional significance of these interactions in the regulation of cell motility is poorly known. Here we have addressed the involvement of the liprin-α1 partner GIT1 in liprin-α1-mediated effects on cell spreading and migration. GIT1 depletion inhibited spreading by affecting the lamellipodia, and prevented liprin-α1-enhanced spreading. Conversely inhibition of the formation of the liprin-α1-GIT complex by expression of liprin-ΔCC3 could still enhance spreading, although to a lesser extent compared to full length liprin-α1. No cumulative effects were observed after depletion of both liprin-α1 and GIT1, suggesting that the two proteins belong to the same signaling network in the regulation of cell spreading. Our data suggest that liprin-α1 may compete with paxillin for binding to GIT1, while binding of βPIX to GIT1 was unaffected by the presence of liprin-α1. Interestingly, GIT and liprin-α1 reciprocally regulated their subcellular localization, since liprin-α1 overexpression, but not the GIT binding-defective liprin-ΔCC3 mutant, affected the localization of endogenous GIT at peripheral and mature central focal adhesions, while the expression of a truncated, active form of GIT1 enhanced the localization of endogenous liprin-α1 at the edge of spreading cells. Moreover, GIT1 was required for liprin-α1-enhanced haptotatic migration, although the direct interaction between liprin-α1 and GIT1 was not needed. Our findings show that the functional interaction between liprin-α1 and GIT1 cooperate in the regulation of integrin-dependent cell spreading and motility on extracellular matrix. These findings and the possible competition of liprin-α1 with paxillin for binding to GIT1 suggest that alternative binding of GIT1 to either liprin-α1 or paxillin plays distinct roles in different phases of the protrusive activity in the cell.
Conflict of interest statement
Figures


References
-
- Le Clainche C, Carlier MF. Regulation of actin assembly associated with protrusion and adhesion in cell migration. Physiol Rev. 2008;88:489–513. - PubMed
-
- Hoefen RJ, Berk BC. The multifunctional GIT family of proteins. J Cell Sci. 2006;119:1469–1475. - PubMed
-
- Manser E, Loo TH, Koh CG, Zhao ZS, Chen XQ, et al. PAK kinases are directly coupled to the PIX family of nucleotide exchange factors. Mol Cell. 1998;1:183–192. - PubMed
-
- Schlenker O, Rittinger K. Structures of dimeric GIT1 and trimeric beta-PIX and implications for GIT-PIX complex assembly. J Mol Biol. 2009;386:280–289. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

