Phos-tag-based analysis of myosin regulatory light chain phosphorylation in human uterine myocytes
- PMID: 21695279
- PMCID: PMC3111472
- DOI: 10.1371/journal.pone.0020903
Phos-tag-based analysis of myosin regulatory light chain phosphorylation in human uterine myocytes
Abstract
Background: The 'phosphate-binding tag' (phos-tag) reagent enables separation of phospho-proteins during SDS-PAGE by impeding migration proportional to their phosphorylation stoichiometry. Western blotting can then be used to detect and quantify the bands corresponding to the phospho-states of a target protein. We present a method for quantification of data regarding phospho-states derived from phos-tag SDS-PAGE. The method incorporates corrections for lane-to-lane loading variability and for the effects of drug vehicles thus enabling the comparison of multiple treatments by using the untreated cellular set-point as a reference. This method is exemplified by quantifying the phosphorylation of myosin regulatory light chain (RLC) in cultured human uterine myocytes.
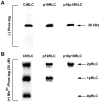
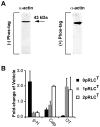
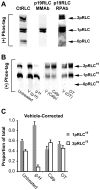
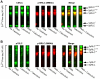
Methodology/principal findings: We have evaluated and validated the concept that, when using an antibody (Ab) against the total-protein, the sum of all phosphorylation states in a single lane represents a 'closed system' since all possible phospho-states and phosphoisotypes are detected. Using this approach, we demonstrate that oxytocin (OT) and calpeptin (Calp) induce RLC kinase (MLCK)- and rho-kinase (ROK)-dependent enhancements in phosphorylation of RLC at T18 and S19. Treatment of myocytes with a phorbol ester (PMA) induced phosphorylation of S1-RLC, which caused a mobility shift in the phos-tag matrices distinct from phosphorylation at S19.
Conclusion/significance: We have presented a method for analysis of phospho-state data that facilitates quantitative comparison to a reference control without the use of a traditional 'loading' or 'reference' standard. This analysis is useful for assessing effects of putative agonists and antagonists where all phospho-states are represented in control and experimental samples. We also demonstrated that phosphorylation of RLC at S1 is inducible in intact uterine myocytes, though the signal in the resting samples was not sufficiently abundant to allow quantification by the approach used here.
Conflict of interest statement
Figures
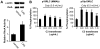

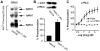
References
-
- Hunter T. Signaling–2000 and beyond. Cell. 2000;100:113–127. - PubMed
-
- Thingholm TE, Jensen ON, Larsen MR. Analytical strategies for phosphoproteomics. Proteomics. 2009;9:1451–1468. - PubMed
-
- Kinoshita E, Kinoshita-Kikuta E, Takiyama K, Koike T. Phosphate-binding tag, a new tool to visualize phosphorylated proteins. Mol Cell Proteomics. 2006;5:749–757. - PubMed
-
- Kinoshita E, Kinoshita-Kikuta E, Koike T. Separation and detection of large phosphoproteins using Phos-tag SDS-PAGE. Nat Protoc. 2009;4:1513–1521. - PubMed
-
- Takeya K, Loutzenhiser K, Shiraishi M, Loutzenhiser R, Walsh MP. A highly sensitive technique to measure myosin regulatory light chain phosphorylation: the first quantification in renal arterioles. Am J Physiol Renal Physiol. 2008;294:F1487–1492. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

