The effect of myosin RLC phosphorylation in normal and cardiomyopathic mouse hearts
- PMID: 21696541
- PMCID: PMC3193868
- DOI: 10.1111/j.1582-4934.2011.01371.x
The effect of myosin RLC phosphorylation in normal and cardiomyopathic mouse hearts
Abstract
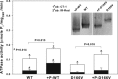
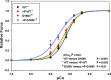
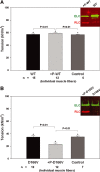
Phosphorylation of the myosin regulatory light chain (RLC) by Ca(2+)-calmodulin-activated myosin light chain kinase (MLCK) is known to be essential for the inotropic function of the heart. In this study, we have examined the effects of MLCK-phosphorylation of transgenic (Tg) mouse cardiac muscle preparations expressing the D166V (aspartic acid to valine)-RLC mutation, identified to cause familial hypertrophic cardiomyopathy with malignant outcomes. Our previous work with Tg-D166V mice demonstrated a large increase in the Ca(2+) sensitivity of contraction, reduced maximal ATPase and force and a decreased level of endogenous RLC phosphorylation. Based on studies demonstrating the beneficial and/or protective effects of cardiac myosin phosphorylation for heart function, we hypothesized that an ex vivo phosphorylation of Tg-D166V cardiac muscle may rescue the detrimental contractile phenotypes observed earlier at the level of single myosin molecules and in Tg-D166V papillary muscle fibres. We showed that MLCK-induced phosphorylation of Tg-D166V cardiac myofibrils and muscle fibres was able to increase the reduced myofibrillar ATPase and reverse an abnormally increased Ca(2+) sensitivity of force to the level observed for Tg-wild-type (WT) muscle. However, in contrast to Tg-WT, which displayed a phosphorylation-induced increase in steady-state force, the maximal tension in Tg-D166V papillary muscle fibres decreased upon phosphorylation. With the exception of force generation data, our results support the notion that RLC phosphorylation works as a rescue mechanism alleviating detrimental functional effects of a disease causing mutation. Further studies are necessary to elucidate the mechanism of this unexpected phosphorylation-induced decrease in maximal tension in Tg-D166V-skinned muscle fibres.
© 2011 The Authors Journal of Cellular and Molecular Medicine © 2011 Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd.
Figures


References
-
- Alcalai R, Seidman JG, Seidman CE. Genetic basis of hypertrophic cardiomyopathy: from bench to the clinics. J Cardiovasc Electrophysiol. 2008;19:104–10. - PubMed
-
- Szczesna D. Regulatory light chains of striated muscle myosin. Structure, function and malfunction. Curr Drug Targets Cardiovasc Haematol Disord. 2003;3:187–97. - PubMed
-
- Poetter K, Jiang H, Hassanzadeh S, et al. Mutations in either the essential or regulatory light chains of myosin are associated with a rare myopathy in human heart and skeletal muscle. Nat Genet. 1996;13:63–9. - PubMed
-
- Richard P, Charron P, Carrier L, et al. Hypertrophic cardiomyopathy: distribution of disease genes, spectrum of mutations, and implications for a molecular diagnosis strategy. Circulation. 2003;107:2227–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

