Vpx rescues HIV-1 transduction of dendritic cells from the antiviral state established by type 1 interferon
- PMID: 21696578
- PMCID: PMC3130655
- DOI: 10.1186/1742-4690-8-49
Vpx rescues HIV-1 transduction of dendritic cells from the antiviral state established by type 1 interferon
Abstract
Background: Vpx is a virion-associated protein encoded by SIVSM, a lentivirus endemic to the West African sooty mangabey (Cercocebus atys). HIV-2 and SIVMAC, zoonoses resulting from SIVSM transmission to humans or Asian rhesus macaques (Macaca mulatta), also encode Vpx. In myeloid cells, Vpx promotes reverse transcription and transduction by these viruses. This activity correlates with Vpx binding to DCAF1 (VPRBP) and association with the DDB1/RBX1/CUL4A E3 ubiquitin ligase complex. When delivered experimentally to myeloid cells using VSV G-pseudotyped virus-like particles (VLPs), Vpx promotes reverse transcription of retroviruses that do not normally encode Vpx.
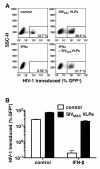
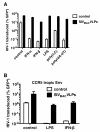
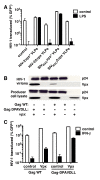
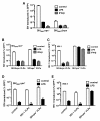
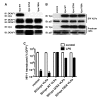
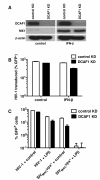
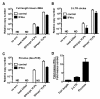
Results: Here we show that Vpx has the extraordinary ability to completely rescue HIV-1 transduction of human monocyte-derived dendritic cells (MDDCs) from the potent antiviral state established by prior treatment with exogenous type 1 interferon (IFN). The magnitude of rescue was up to 1,000-fold, depending on the blood donor, and was also observed after induction of endogenous IFN and IFN-stimulated genes (ISGs) by LPS, poly(I:C), or poly(dA:dT). The effect was relatively specific in that Vpx-associated suppression of soluble IFN-β production, of mRNA levels for ISGs, or of cell surface markers for MDDC differentiation, was not detected. Vpx did not rescue HIV-2 or SIVMAC transduction from the antiviral state, even in the presence of SIVMAC or HIV-2 VLPs bearing additional Vpx, or in the presence of HIV-1 VLPs bearing all accessory genes. In contrast to the effect of Vpx on transduction of untreated MDDCs, HIV-1 rescue from the antiviral state was not dependent upon Vpx interaction with DCAF1 or on the presence of DCAF1 within the MDDC target cells. Additionally, although Vpx increased the level of HIV-1 reverse transcripts in MDDCs to the same extent whether or not MDDCs were treated with IFN or LPS, Vpx rescued a block specific to the antiviral state that occurred after HIV-1 cDNA penetrated the nucleus.
Conclusion: Vpx provides a tool for the characterization of a potent, new HIV-1 restriction activity, which acts in the nucleus of type 1 IFN-treated dendritic cells.
Figures
Similar articles
-
Vpx rescue of HIV-1 from the antiviral state in mature dendritic cells is independent of the intracellular deoxynucleotide concentration.Retrovirology. 2014 Feb 1;11:12. doi: 10.1186/1742-4690-11-12. Retrovirology. 2014. PMID: 24485168 Free PMC article.
-
Lentiviral Vpx accessory factor targets VprBP/DCAF1 substrate adaptor for cullin 4 E3 ubiquitin ligase to enable macrophage infection.PLoS Pathog. 2008 May 9;4(5):e1000059. doi: 10.1371/journal.ppat.1000059. PLoS Pathog. 2008. PMID: 18464893 Free PMC article.
-
Interferon block to HIV-1 transduction in macrophages despite SAMHD1 degradation and high deoxynucleoside triphosphates supply.Retrovirology. 2013 Mar 11;10:30. doi: 10.1186/1742-4690-10-30. Retrovirology. 2013. PMID: 23497353 Free PMC article.
-
SAMHD1: a novel antiviral factor in intrinsic immunity.Future Microbiol. 2012 Sep;7(9):1117-26. doi: 10.2217/fmb.12.81. Future Microbiol. 2012. PMID: 22953710 Review.
-
Antiviral activity of the interferon-induced cellular protein BST-2/tetherin.AIDS Res Hum Retroviruses. 2009 Dec;25(12):1197-210. doi: 10.1089/aid.2009.0253. AIDS Res Hum Retroviruses. 2009. PMID: 19929170 Free PMC article. Review.
Cited by
-
SAMHD1 restricts HIV-1 cell-to-cell transmission and limits immune detection in monocyte-derived dendritic cells.J Virol. 2013 Mar;87(5):2846-56. doi: 10.1128/JVI.02514-12. Epub 2012 Dec 26. J Virol. 2013. PMID: 23269793 Free PMC article.
-
Differential effects of Vpr on single-cycle and spreading HIV-1 infections in CD4+ T-cells and dendritic cells.PLoS One. 2012;7(5):e35385. doi: 10.1371/journal.pone.0035385. Epub 2012 May 3. PLoS One. 2012. PMID: 22570689 Free PMC article.
-
Access of HIV-2 to CD169-dependent dendritic cell-mediated trans infection pathway is attenuated.Virology. 2016 Oct;497:328-336. doi: 10.1016/j.virol.2016.07.029. Epub 2016 Aug 11. Virology. 2016. PMID: 27521724 Free PMC article.
-
Cullin4A and cullin4B are interchangeable for HIV Vpr and Vpx action through the CRL4 ubiquitin ligase complex.J Virol. 2014 Jun;88(12):6944-58. doi: 10.1128/JVI.00241-14. Epub 2014 Apr 9. J Virol. 2014. PMID: 24719410 Free PMC article.
-
Evidence for IFNα-induced, SAMHD1-independent inhibitors of early HIV-1 infection.Retrovirology. 2013 Feb 25;10:23. doi: 10.1186/1742-4690-10-23. Retrovirology. 2013. PMID: 23442224 Free PMC article.
References
-
- Hirsch VM, Sharkey ME, Brown CR, Brichacek B, Goldstein S, Wakefield J, Byrum R, Elkins WR, Hahn BH, Lifson JD, Stevenson M. Vpx is required for dissemination and pathogenesis of SIV(SM) PBj: evidence of macrophage-dependent viral amplification. Nat Med. 1998;4:1401–1408. doi: 10.1038/3992. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

