Sodium arsenite and hyperthermia modulate cisplatin-DNA damage responses and enhance platinum accumulation in murine metastatic ovarian cancer xenograft after hyperthermic intraperitoneal chemotherapy (HIPEC)
- PMID: 21696631
- PMCID: PMC3143084
- DOI: 10.1186/1757-2215-4-9
Sodium arsenite and hyperthermia modulate cisplatin-DNA damage responses and enhance platinum accumulation in murine metastatic ovarian cancer xenograft after hyperthermic intraperitoneal chemotherapy (HIPEC)
Abstract
Background: Epithelial ovarian cancer (EOC) is the leading cause of gynecologic cancer death in the USA. Recurrence rates are high after front-line therapy and most patients eventually die from platinum (Pt) - resistant disease. Cisplatin resistance is associated with increased nucleotide excision repair (NER), decreased mismatch repair (MMR) and decreased platinum uptake. The objective of this study is to investigate how a novel combination of sodium arsenite (NaAsO2) and hyperthermia (43°C) affect mechanisms of cisplatin resistance in ovarian cancer.
Methods: We established a murine model of metastatic EOC by intraperitoneal injection of A2780/CP70 human ovarian cancer cells into nude mice. We developed a murine hyperthermic intraperitoneal chemotherapy model to treat the mice. Mice with peritoneal metastasis were perfused for 1 h with 3 mg/kg cisplatin ± 26 mg/kg NaAsO2 at 37 or 43°C. Tumors and tissues were collected at 0 and 24 h after treatment.
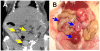
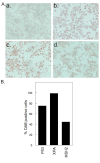
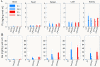
Results: Western blot analysis of p53 and key NER proteins (ERCC1, XPC and XPA) and MMR protein (MSH2) suggested that cisplatin induced p53, XPC and XPA and suppressed MSH2 consistent with resistant phenotype. Hyperthermia suppressed cisplatin-induced XPC and prevented the induction of XPA by cisplatin, but it had no effect on Pt uptake or retention in tumors. NaAsO2 prevented XPC induction by cisplatin; it maintained higher levels of MSH2 in tumors and enhanced initial accumulation of Pt in tumors. Combined NaAsO2 and hyperthermia decreased cisplatin-induced XPC 24 h after perfusion, maintained higher levels of MSH2 in tumors and significantly increased initial accumulation of Pt in tumors. ERCC1 levels were generally low except for NaAsO2 co-treatment with cisplatin. Systemic Pt and arsenic accumulation for all treatment conditions were in the order: kidney > liver = spleen > heart > brain and liver > kidney = spleen > heart > brain respectively. Metal levels generally decreased in systemic tissues within 24 h after treatment.
Conclusion: NaAsO2 and/or hyperthermia have the potential to sensitize tumors to cisplatin by inhibiting NER, maintaining functional MMR and enhancing tumor platinum uptake.
Figures



References
-
- Ozols RF. Treatment goals in ovarian cancer. Int J Gynecol Cancer. 2005;15(Suppl 1):3–11. - PubMed
-
- van d V, van d V, Zoetmulder FA, van Goethem AR, van T O, ten Bokkel Huinink WW, Beijnen JH, Bartelink H, Begg AC. Intraperitoneal cisplatin with regional hyperthermia in advanced ovarian cancer: pharmacokinetics and cisplatin-DNA adduct formation in patients and ovarian cancer cell lines. Eur J Cancer. 1998;34:148–154. doi: 10.1016/S0959-8049(97)00370-5. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

