Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS®): depression, anxiety, and anger
- PMID: 21697139
- PMCID: PMC3153635
- DOI: 10.1177/1073191111411667
Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS®): depression, anxiety, and anger
Abstract
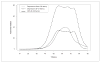
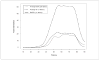
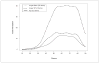
The authors report on the development and calibration of item banks for depression, anxiety, and anger as part of the Patient-Reported Outcomes Measurement Information System (PROMIS®). Comprehensive literature searches yielded an initial bank of 1,404 items from 305 instruments. After qualitative item analysis (including focus groups and cognitive interviewing), 168 items (56 for each construct) were written in a first person, past tense format with a 7-day time frame and five response options reflecting frequency. The calibration sample included nearly 15,000 respondents. Final banks of 28, 29, and 29 items were calibrated for depression, anxiety, and anger, respectively, using item response theory. Test information curves showed that the PROMIS item banks provided more information than conventional measures in a range of severity from approximately -1 to +3 standard deviations (with higher scores indicating greater distress). Short forms consisting of seven to eight items provided information comparable to legacy measures containing more items.
Conflict of interest statement
Figures

References
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. Washington, DC: Author; 2000.
-
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: Psychometric properties. Journal of Consulting and Clinical Psychology. 1988;56:893–897. - PubMed
-
- Bejar II. An application of the continuous response level model to personality measurement. Applied Psychological Measurement. 1977;1:509–521.
-
- Berzon R, Patrick D, Guyatt G, Conley JM. Intellectual property considerations in the development and use of HRQL measures for clinical trial research. Quality of Life Research. 1994;3:273–277. - PubMed
Publication types
MeSH terms
Grants and funding
- U01AR52155/AR/NIAMS NIH HHS/United States
- U01 AR052181/AR/NIAMS NIH HHS/United States
- U01AR52170/AR/NIAMS NIH HHS/United States
- U01AR52158/AR/NIAMS NIH HHS/United States
- U01AR52171/AR/NIAMS NIH HHS/United States
- U01 AR052155/AR/NIAMS NIH HHS/United States
- U01AR52186/AR/NIAMS NIH HHS/United States
- U01 AR052171/AR/NIAMS NIH HHS/United States
- U01 AR052186/AR/NIAMS NIH HHS/United States
- UL1 TR000005/TR/NCATS NIH HHS/United States
- U01 AR052170/AR/NIAMS NIH HHS/United States
- U01 AR052177/AR/NIAMS NIH HHS/United States
- U01AR52177/AR/NIAMS NIH HHS/United States
- U01AR52181/AR/NIAMS NIH HHS/United States
- U01 AR052158/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

